Modulation of orphan nuclear receptor Nur77-mediated apoptotic pathway by acetylshikonin and analogues
- PMID: 18974131
- PMCID: PMC2679687
- DOI: 10.1158/0008-5472.CAN-08-1972
Modulation of orphan nuclear receptor Nur77-mediated apoptotic pathway by acetylshikonin and analogues
Abstract
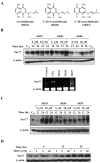
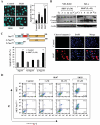
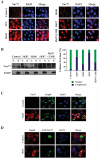
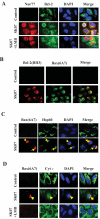
Shikonin derivatives, which are the active components of the medicinal plant Lithospermum erythrorhizon, exhibit many biological effects including apoptosis induction through undefined mechanisms. We recently discovered that orphan nuclear receptor Nur77 migrates from the nucleus to the mitochondria, where it binds to Bcl-2 to induce apoptosis. Here, we report that certain shikonin derivatives could modulate the Nur77/Bcl-2 apoptotic pathway by increasing levels of Nur77 protein and promoting its mitochondrial targeting in cancer cells. Structural modification of acetylshikonin resulted in the identification of a derivative 5,8-diacetoxyl-6-(1'-acetoxyl-4'-methyl-3'-pentenyl)-1,4-naphthaquinones (SK07) that exhibited improved efficacy and specificity in activating the pathway. Unlike other Nur77 modulators, shikonins increased the levels of Nur77 protein through their posttranscriptional regulation. The apoptotic effect of SK07 was impaired in Nur77 knockout cells and suppressed by cotreatment with leptomycin B that inhibited Nur77 cytoplasmic localization. Furthermore, SK07 induced apoptosis in cells expressing the COOH-terminal half of Nur77 protein but not its NH(2)-terminal region. Our data also showed that SK07-induced apoptosis was associated with a Bcl-2 conformational change and Bax activation. Together, our results show that certain shikonin derivatives act as modulators of the Nur77-mediated apoptotic pathway and identify a new shikonin-based lead that targets Nur77 for apoptosis induction.
Figures


References
-
- Zhang XK. Targeting Nur77 translocation. Expert Opin Ther Tar. 2007;11:69–79. - PubMed
-
- Moll UM, Marchenko N, Zhang XK. p53 and Nur77/TR3 - transcription factors that directly target mitochondria for cell death induction. Oncogene. 2006;25:4725–43. - PubMed
-
- Chen GQ, Lin B, Dawson MI, Zhang XK. Nicotine modulates the effects of retinoids on growth inhibition and RAR beta expression in lung cancer cells. Int J Cancer. 2002;99:171–8. - PubMed
-
- Wu Q, Liu S, Ye XF, Huang ZW, Su WJ. Dual roles of Nur77 in selective regulation of apoptosis and cell cycle by TPA and ATRA in gastric cancer cells. Carcinogenesis. 2002;23:1583–92. - PubMed
-
- Stasik I, Rapak A, Kalas W, Ziolo E, Strzadala L. Ionomycin-induced apoptosis of thymocytes is independent of Nur77 NBRE or NurRE binding, but is accompanied by Nur77 mitochondrial targeting. BBA-Mol Cell Res. 2007;1773:1483–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

