Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer
- PMID: 18974148
- PMCID: PMC2584362
- DOI: 10.1158/0008-5472.CAN-08-2494
Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer
Abstract
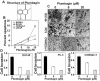
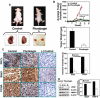
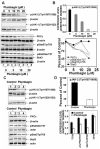
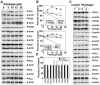
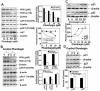
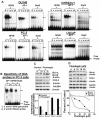
Prostate cancer (PCa) is the second leading cause of cancer-related deaths in men. Hormone-refractory invasive PCa is the end stage and accounts for the majority of PCa patient deaths. We present here that plumbagin (PL), a quinoid constituent isolated from the root of the medicinal plant Plumbago zeylanica L., may be a potential novel agent in the control of hormone-refractory PCa. Specific observations are the findings that PL inhibited PCa cell invasion and selectively induced apoptosis in PCa cells but not in immortalized nontumorigenic prostate epithelial RWPE-1 cells. In addition, i.p. administration of PL (2 mg/kg body weight), beginning 3 days after ectopic implantation of hormone-refractory DU145 PCa cells, delayed tumor growth by 3 weeks and reduced both tumor weight and volume by 90%. Discontinuation of PL treatment in PL-treated mice for as long as 4 weeks did not result in progression of tumor growth. PL, at concentrations as low as 5 micromol/L, inhibited in both cultured PCa cells and DU145 xenografts (a) the expression of protein kinase Cepsilon (PKCepsilon), phosphatidylinositol 3-kinase, phosphorylated AKT, phosphorylated Janus-activated kinase-2, and phosphorylated signal transducer and activator of transcription 3 (Stat3); (b) the DNA-binding activity of transcription factors activator protein-1, nuclear factor-kappaB, and Stat3; and (c) Bcl-xL, cdc25A, and cyclooxygenase-2 expression. The results indicate for the first time, using both in vitro and in vivo preclinical models, that PL inhibits the growth and invasion of PCa. PL inhibits multiple molecular targets including PKCepsilon, a predictive biomarker of PCa aggressiveness. PL may be a novel agent for therapy of hormone-refractory PCa.
Figures
References
-
- Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 2: Androgen-receptor cofactors and bypass pathways. BJU Int. 2005;95:1327–35. - PubMed
-
- Zhou J, Scholes J, Hsieh JT. Signal transduction targets in androgen-independent prostate cancer. Cancer Metastasis Rev. 2001;20:351–62. - PubMed
-
- Silvestris N, Leone B, Numico G, et al. Present status and perspectives in the treatment of hormone-refractory prostate cancer. Oncology. 2005;69:273–82. - PubMed
-
- Chau CH, Figg WD. Molecular and phenotypic heterogeneity of metastatic prostate cancer. Cancer Biol Ther. 2005;4:166–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

