Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA
- PMID: 18974154
- PMCID: PMC2597081
- DOI: 10.1158/0008-5472.CAN-08-2397
Targeting melanoma growth and metastasis with systemic delivery of liposome-incorporated protease-activated receptor-1 small interfering RNA
Abstract
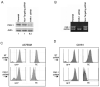
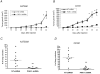
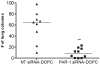
The thrombin receptor [protease-activated receptor-1 (PAR-1)] is overexpressed in highly metastatic melanoma cell lines and in patients with metastatic lesions. Activation of PAR-1 leads to cell signaling and up-regulation of genes involved in adhesion, invasion, and angiogenesis. Herein, we stably silence PAR-1 through the use of lentiviral short hairpin RNA and found significant decreases in both tumor growth (P < 0.01) and metastasis (P < 0.001) of highly metastatic melanoma cell lines in vivo. The use of viruses for therapy is not ideal as it can induce toxic immune responses and possible gene alterations following viral integration. Therefore, we also used systemic delivery of PAR-1 small interfering RNA (siRNA) incorporated into neutral liposomes [1,2-dioleoyl-sn-glycero-3-phosphatidylcholine (DOPC)] to decrease melanoma growth and metastasis in vivo. Significant decreases in tumor growth, weight, and metastatic lung colonies (P < 0.001 for all) were found in mice treated with PAR-1 siRNA-DOPC. The in vivo effects of PAR-1 on invasion and angiogenesis were analyzed via immunohistochemistry. Concomitant decreases in vascular endothelial growth factor, interleukin-8, and matrix metalloproteinase-2 expression levels, as well as decreased blood vessel density (CD31), were found in tumor samples from PAR-1 siRNA-treated mice, suggesting that PAR-1 is a regulator of melanoma cell growth and metastasis by affecting angiogenic and invasive factors. We propose that siRNA incorporated into DOPC nanoparticles could be delivered systemically and used as a new modality for melanoma treatment.
Figures



Similar articles
-
Focal adhesion kinase targeting using in vivo short interfering RNA delivery in neutral liposomes for ovarian carcinoma therapy.Clin Cancer Res. 2006 Aug 15;12(16):4916-24. doi: 10.1158/1078-0432.CCR-06-0021. Clin Cancer Res. 2006. PMID: 16914580 Free PMC article.
-
Up-regulation of Flotillin-2 is associated with melanoma progression and modulates expression of the thrombin receptor protease activated receptor 1.Cancer Res. 2004 Oct 15;64(20):7361-9. doi: 10.1158/0008-5472.CAN-04-0823. Cancer Res. 2004. PMID: 15492257
-
Protease activated receptor-1 inhibits the Maspin tumor-suppressor gene to determine the melanoma metastatic phenotype.Proc Natl Acad Sci U S A. 2011 Jan 11;108(2):626-31. doi: 10.1073/pnas.1006886108. Epub 2010 Dec 27. Proc Natl Acad Sci U S A. 2011. PMID: 21187389 Free PMC article.
-
The emerging role of the thrombin receptor (PAR-1) in melanoma metastasis--a possible therapeutic target.Oncotarget. 2011 Jan-Feb;2(1-2):8-17. doi: 10.18632/oncotarget.211. Oncotarget. 2011. PMID: 21378407 Free PMC article. Review.
-
PAR-1 and thrombin: the ties that bind the microenvironment to melanoma metastasis.Cancer Res. 2011 Nov 1;71(21):6561-6. doi: 10.1158/0008-5472.CAN-11-1432. Epub 2011 Oct 18. Cancer Res. 2011. PMID: 22009534 Free PMC article. Review.
Cited by
-
The multifaceted role of CD146/MCAM in the promotion of melanoma progression.Cancer Cell Int. 2015 Feb 4;15(1):3. doi: 10.1186/s12935-014-0147-z. eCollection 2015. Cancer Cell Int. 2015. PMID: 25685061 Free PMC article.
-
PAR1 signaling on tumor cells limits tumor growth by maintaining a mesenchymal phenotype in pancreatic cancer.Oncotarget. 2018 Aug 10;9(62):32010-32023. doi: 10.18632/oncotarget.25880. eCollection 2018 Aug 10. Oncotarget. 2018. PMID: 30174793 Free PMC article.
-
Reactive astrocytes protect melanoma cells from chemotherapy by sequestering intracellular calcium through gap junction communication channels.Neoplasia. 2010 Sep;12(9):748-54. doi: 10.1593/neo.10602. Neoplasia. 2010. PMID: 20824051 Free PMC article.
-
Targeting GPCRs and Their Signaling as a Therapeutic Option in Melanoma.Cancers (Basel). 2022 Jan 29;14(3):706. doi: 10.3390/cancers14030706. Cancers (Basel). 2022. PMID: 35158973 Free PMC article. Review.
-
Delivery of intracellular-acting biologics in pro-apoptotic therapies.Curr Pharm Des. 2011;17(3):293-319. doi: 10.2174/138161211795049642. Curr Pharm Des. 2011. PMID: 21348831 Free PMC article. Review.
References
-
- Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–68. - PubMed
-
- Even-Ram SC, Maoz M, Pokroy E, et al. Tumor cell invasion is promoted by activation of protease activated receptor-1 in cooperation with the alpha vbeta 5 integrin. J Biol Chem. 2001;276:10952–62. - PubMed
-
- Wojtukiewicz MZ, Tang DG, Nelson KK, Walz DA, Diglio CA, Honn KV. Thrombin enhances tumor cell adhesive and metastatic properties via increased alpha IIb beta 3 expression on the cell surface. Thromb Res. 1992;68:233–45. - PubMed
-
- Senger DR, Ledbetter SR, Claffey KP, Papadopoulos-Sergiou A, Peruzzi CA, Detmar M. Stimulation of endothelial cell migration by vascular permeability factor/vascular endothelial growth factor through cooperative mechanisms involving the alphavbeta3 integrin, osteopontin, and thrombin. Am J Pathol. 1996;149:293–305. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

