Glucose-oxidized low-density lipoproteins enhance insulin-like growth factor I-stimulated smooth muscle cell proliferation by inhibiting integrin-associated protein cleavage
- PMID: 18974270
- PMCID: PMC5393262
- DOI: 10.1210/en.2008-1090
Glucose-oxidized low-density lipoproteins enhance insulin-like growth factor I-stimulated smooth muscle cell proliferation by inhibiting integrin-associated protein cleavage
Erratum in
- Endocrinology. 2010 Jun;151(6):2967
Abstract
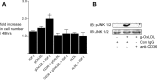
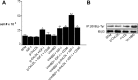
Prior published reports have demonstrated that glucose-oxidized low-density lipoproteins (g-OxLDL) enhance the proliferative response of vascular smooth muscle cells (SMC) to IGF-I. Our previous studies have determined that the regulation of cleavage of integrin-associated protein (IAP) by matrix-metalloprotease-2 (MMP-2) in diabetic mice in response to hyperglycemia is a key regulator of the response of SMC to IGF-I. Because chronic hyperglycemia enhances glucose-induced LDL oxidation, these studies were conducted to determine whether g-OxLDL modulates the response of SMC to IGF-I by regulating MMP-2-mediated cleavage of IAP. We determined that exposure of SMC to g-OxLDL, but not native LDL, was sufficient to facilitate an increase in cell proliferation in response to IGF-I. Exposure to an anti-CD36 antibody, which has been shown to inhibit g-OxLDL-mediated signaling, inhibited the effects of g-OxLDL on IGF-I-stimulated SMC proliferation. The effect of g-OxLDL could be attributed, in part, to an associated decrease in proteolytic cleavage of IAP leading to increase in the basal association between IAP and Src homology 2 domain-containing protein tyrosine phosphatase substrate-1, which is required for IGF-I-stimulated proliferation. The inhibitory effect of g-OxLDL on IAP cleavage appeared to be due to its ability to decrease the amount of activated MMP-2, the protease responsible for IAP cleavage. In conclusion, these data provide a molecular mechanism to explain previous studies that have reported an enhancing effect of g-OxLDL on IGF-I-stimulated SMC proliferation.
Figures




References
-
- Kannel WB, McGee DL 1979. Diabetes and cardiovascular risk factors: the Framingham study. Circulation 59:8–13 - PubMed
-
- Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, Gatling W, Bingley PJ, Patterson CC 2003. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia 46:760–765 - PubMed
-
- Ross R 1993. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

