Copper-induced translocation of the Wilson disease protein ATP7B independent of Murr1/COMMD1 and Rab7
- PMID: 18974300
- PMCID: PMC2626389
- DOI: 10.2353/ajpath.2008.071134
Copper-induced translocation of the Wilson disease protein ATP7B independent of Murr1/COMMD1 and Rab7
Abstract
Wilson disease is a genetic disorder of copper metabolism. Impaired biliary excretion results in a gradual accumulation of copper, which leads to severe disease. The specific gene defect lies in the Wilson disease protein, ATP7B, a copper-transporting ATPase that is highly active in hepatocytes. The two major functions of ATP7B in the liver are the copper loading of ceruloplasmin in the Golgi apparatus, and the excretion of excess copper into the bile. In response to elevated copper levels, ATP7B shows a unique intracellular trafficking pattern that is required for copper excretion from the Golgi apparatus into dispersed vesicles. We analyzed the translocation of ATP7B by both confocal microscopy and RNA interference, testing current models that suggest the involvement of Murr1/COMMD1 and Rab7 in this pathway. We found that although the ATP7B translocation is conserved among nonhepatic cell lines, there is no co-localization with Murr1/COMMD1 or the Rab marker proteins of the endolysosomal system. Consistent with this finding, the translocation of ATP7B was not impaired by the depletion of either Murr1/COMMD1 or Rab7, or by a dominant-negative Rab7 mutant. In conclusion, our data suggest that the translocation of ATP7B takes place independently of Rab7-regulated endosomal traffic events. Murr1/COMMD1 plays a role in a later step of the copper excretion pathway but is not involved in the translocation of the Wilson disease protein.
Figures
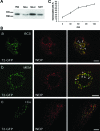
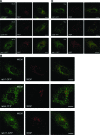
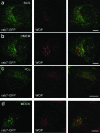
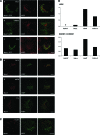

Similar articles
-
Niemann-Pick C1 protein transports copper to the secretory compartment from late endosomes where ATP7B resides.Exp Cell Res. 2009 Jan 15;315(2):119-26. doi: 10.1016/j.yexcr.2008.10.022. Epub 2008 Oct 31. Exp Cell Res. 2009. PMID: 19007772
-
The Wilson disease protein ATP7B resides in the late endosomes with Rab7 and the Niemann-Pick C1 protein.Am J Pathol. 2005 Feb;166(2):499-510. doi: 10.1016/S0002-9440(10)62272-9. Am J Pathol. 2005. PMID: 15681833 Free PMC article.
-
Distinct Wilson's disease mutations in ATP7B are associated with enhanced binding to COMMD1 and reduced stability of ATP7B.Gastroenterology. 2007 Oct;133(4):1316-26. doi: 10.1053/j.gastro.2007.07.020. Epub 2007 Jul 25. Gastroenterology. 2007. PMID: 17919502 Free PMC article.
-
Functional understanding of the versatile protein copper metabolism MURR1 domain 1 (COMMD1) in copper homeostasis.Ann N Y Acad Sci. 2014 May;1314:6-14. doi: 10.1111/nyas.12353. Epub 2014 Feb 12. Ann N Y Acad Sci. 2014. PMID: 24697840 Review.
-
Hepatic copper-transporting ATPase ATP7B: function and inactivation at the molecular and cellular level.Biometals. 2007 Jun;20(3-4):627-37. doi: 10.1007/s10534-006-9074-3. Epub 2007 Feb 1. Biometals. 2007. PMID: 17268820 Review.
Cited by
-
Epidemiology of Wilson's Disease and Pathogenic Variants of the ATP7B Gene Leading to Diversified Protein Disfunctions.Int J Mol Sci. 2024 Feb 18;25(4):2402. doi: 10.3390/ijms25042402. Int J Mol Sci. 2024. PMID: 38397079 Free PMC article. Review.
-
Genetic analysis of BIRC4/XIAP as a putative modifier gene of Wilson disease.J Inherit Metab Dis. 2010 Dec;33 Suppl 3:S233-40. doi: 10.1007/s10545-010-9123-5. Epub 2010 Jun 2. J Inherit Metab Dis. 2010. PMID: 20517649
-
Wilson Disease: Copper-Mediated Cuproptosis, Iron-Related Ferroptosis, and Clinical Highlights, with Comprehensive and Critical Analysis Update.Int J Mol Sci. 2024 Apr 26;25(9):4753. doi: 10.3390/ijms25094753. Int J Mol Sci. 2024. PMID: 38731973 Free PMC article. Review.
-
Decreased serum antioxidant capacity in patients with Wilson disease is associated with neurological symptoms.J Inherit Metab Dis. 2012 May;35(3):541-8. doi: 10.1007/s10545-011-9422-5. Epub 2011 Dec 3. J Inherit Metab Dis. 2012. PMID: 22139496
-
COMMD1 is linked to the WASH complex and regulates endosomal trafficking of the copper transporter ATP7A.Mol Biol Cell. 2015 Jan 1;26(1):91-103. doi: 10.1091/mbc.E14-06-1073. Epub 2014 Oct 29. Mol Biol Cell. 2015. PMID: 25355947 Free PMC article.
References
-
- Wijmenga C, Klomp LW. Molecular regulation of copper excretion in the liver. Proc Nutr Soc. 2004;63:31–39. - PubMed
-
- Prohaska JR, Gybina AA. Intracellular copper transport in mammals. J Nutr. 2004;134:1003–1006. - PubMed
-
- Gitlin JD. Wilson disease. Gastroenterology. 2003;125:1868–1877. - PubMed
-
- Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson’s disease. Lancet. 2007;369:397–408. - PubMed
-
- Bull PC, Thomas GR, Rommens JM, Forbes JR, Cox DW. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993;5:327–337. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

