Serum amyloid A, but not C-reactive protein, stimulates vascular proteoglycan synthesis in a pro-atherogenic manner
- PMID: 18974302
- PMCID: PMC2626400
- DOI: 10.2353/ajpath.2008.080201
Serum amyloid A, but not C-reactive protein, stimulates vascular proteoglycan synthesis in a pro-atherogenic manner
Abstract
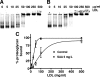
Inflammatory markers serum amyloid A (SAA) and C-reactive protein (CRP) are predictive of cardiac disease and are proposed to play causal roles in the development of atherosclerosis, in which the retention of lipoproteins by vascular wall proteoglycans is critical. The purpose of this study was to determine whether SAA and/or CRP alters vascular proteoglycan synthesis and lipoprotein retention in a pro-atherogenic manner. Vascular smooth muscle cells were stimulated with either SAA or CRP (1 to 100 mg/L) and proteoglycans were then isolated and characterized. SAA, but not CRP, increased proteoglycan sulfate incorporation by 50 to 100% in a dose-dependent manner (P < 0.0001), increased glycosaminoglycan chain length, and increased low-density lipoprotein (LDL) binding affinity (K(d), 29 microg/ml LDL versus 90 microg/ml LDL for SAA versus control proteoglycans; P < 0.005). Furthermore, SAA up-regulated biglycan via the induction of endogenous transforming growth factor (TGF)-beta. To determine whether SAA stimulated proteoglycan synthesis in vivo, ApoE(-/-) mice were injected with an adenovirus expressing human SAA-1, a null virus, or saline. Mice that received adenovirus expressing SAA had increased TGF-beta concentrations in plasma and increased aortic biglycan content compared with mice that received either null virus or saline. Thus, SAA alters vascular proteoglycans in a pro-atherogenic manner via the stimulation of TGF-beta and may play a causal role in the development of atherosclerosis.
Figures




References
-
- McLaughlin T, Abbasi F, Lamendola C, Liang L, Reaven G, Schaaf P, Reaven P. Differentiation between obesity and insulin resistance in the association with C-reactive protein. Circulation. 2002;106:2908–2912. - PubMed
-
- Lemieux I, Pascot A, Prud'homme D, Almeras N, Bogaty P, Nadeau A, Bergeron J, Despres JP. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21:961–967. - PubMed
-
- Fröhlich M, Imhof A, Berg G, Hutchinson WL, Pepys MB, Boeing H, Muche R, Brenner H, Koenig W. Association between C-reactive protein and features of the metabolic syndrome: a population-based study. Diabetes Care. 2000;23:1835–1839. - PubMed
-
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. - PubMed
-
- Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

