Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model
- PMID: 18974303
- PMCID: PMC2626378
- DOI: 10.2353/ajpath.2008.080337
Immunological mechanisms underlying the genetic predisposition to severe Staphylococcus aureus infection in the mouse model
Abstract
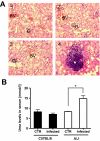
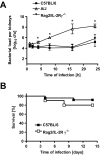
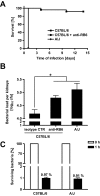
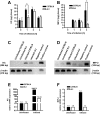
Host genetic variations play a significant role in conferring predisposition to infection. In this study, we examined the immune mechanisms underlying the host genetic predisposition to severe Staphylococcus aureus infection in different mouse strains. Whereas C57BL/6 mice were the most resistant in terms of control of bacterial growth and survival, A/J, DBA/2, and BALB/c mice were highly susceptible and succumbed to infection shortly after bacterial inoculation. Other strains (C3H/HeN, CBA, and C57BL/10) exhibited intermediate susceptibility levels. Susceptibility of mice to S. aureus was associated with an inability to limit bacterial growth in the kidneys and development of pathology. Resistance to S. aureus in C57BL/6 mice was dependent on innate immune mechanisms because Rag2-IL2Rgamma(-/-) C57BL/6 mice, which are deficient in B, T, and NK cells, were also resistant to infection. Indeed, neutrophil depletion or inhibition of neutrophil recruitment rendered C57BL/6 mice completely susceptible to S. aureus, indicating that neutrophils are essential for the observed resistance. Although neutrophil function is not inhibited in A/J mice, expression of neutrophil chemoattractants KC and MIP-2 peaked earlier in the kidneys of C57BL/6 mice than in A/J mice, indicating that a delay in neutrophil recruitment to the site of infection may underlie the increased susceptibility of A/J mice to S. aureus.
Figures




References
-
- Chang FY. Staphylococcus aureus bacteremia and endocarditis. J Microbiol Immunol Infect. 2000;33:63–68. - PubMed
-
- Pittet D, Wenzel RP. Nosocomial bloodstream infections. Secular trends in rates, mortality, and contribution to total hospital deaths. Arch Intern Med. 1995;155:1177–1184. - PubMed
-
- Lautenschlager S, Herzog C, Zimmerli W. Course and outcome of bacteremia due to Staphylococcus aureus: evaluation of different clinical case definitions. Clin Infect Dis. 1993;16:567–573. - PubMed
-
- Bone R. How Gram-positive organisms cause sepsis. J Crit Care. 1993;8:51–59. - PubMed
-
- Tarkowski A, Wagner H. Arthritis and sepsis caused by Staphylococcus aureus: can the tissue injury be reduced by modulating the host’s immune system? Mol Med Today. 1998;4:15–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

