After injection into the striatum, in vitro-differentiated microglia- and bone marrow-derived dendritic cells can leave the central nervous system via the blood stream
- PMID: 18974305
- PMCID: PMC2626379
- DOI: 10.2353/ajpath.2008.080234
After injection into the striatum, in vitro-differentiated microglia- and bone marrow-derived dendritic cells can leave the central nervous system via the blood stream
Abstract
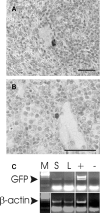
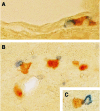
The prototypic migratory trail of tissue-resident dendritic cells (DCs) is via lymphatic drainage. Since the central nervous system (CNS) lacks classical lymphatic vessels, and antigens and cells injected into both the CNS and cerebrospinal fluid have been found in deep cervical lymph nodes, it was thought that CNS-derived DCs exclusively used the cerebrospinal fluid pathway to exit from tissues. It has become evident, however, that DCs found in peripheral organs can also leave tissues via the blood stream. To study whether DCs derived from microglia and bone marrow can also use this route of emigration from the CNS, we performed a series of experiments in which we injected genetically labeled DCs into the striata of rats. We show here that these cells migrated from the injection site to the perivascular space, integrated into the endothelial lining of the CNS vasculature, and were then present in the lumen of CNS blood vessels days after the injection. Moreover, we also found these cells in both mesenteric lymph nodes and spleens. Hence, microglia- and bone marrow-derived DCs can leave the CNS via the blood stream.
Figures


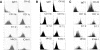



Similar articles
-
Cerebrospinal fluid dendritic cells infiltrate the brain parenchyma and target the cervical lymph nodes under neuroinflammatory conditions.PLoS One. 2008 Oct 2;3(10):e3321. doi: 10.1371/journal.pone.0003321. PLoS One. 2008. PMID: 18830405 Free PMC article.
-
Rat bone marrow progenitor cells transduced in situ by rSV40 vectors differentiate into multiple central nervous system cell lineages.Stem Cells. 2006 Dec;24(12):2801-9. doi: 10.1634/stemcells.2006-0124. Epub 2006 Sep 7. Stem Cells. 2006. PMID: 16960137
-
Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia.FASEB J. 2004 Jun;18(9):998-1000. doi: 10.1096/fj.04-1517fje. Epub 2004 Apr 14. FASEB J. 2004. PMID: 15084516
-
From bone marrow to microglia: barriers and avenues.Trends Immunol. 2008 May;29(5):227-34. doi: 10.1016/j.it.2008.01.010. Epub 2008 Apr 7. Trends Immunol. 2008. PMID: 18396103 Review.
-
CNS dendritic cells: critical participants in CNS inflammation?Neurochem Int. 2006 Jul;49(2):195-203. doi: 10.1016/j.neuint.2006.04.004. Epub 2006 May 30. Neurochem Int. 2006. PMID: 16730862 Review.
Cited by
-
To the Brain and Back: Migratory Paths of Dendritic Cells in Multiple Sclerosis.J Neuropathol Exp Neurol. 2018 Mar 1;77(3):178-192. doi: 10.1093/jnen/nlx114. J Neuropathol Exp Neurol. 2018. PMID: 29342287 Free PMC article. Review.
-
Tryps and trips: cell trafficking across the 100-year-old blood-brain barrier.Trends Neurosci. 2014 Jun;37(6):325-33. doi: 10.1016/j.tins.2014.03.007. Epub 2014 Apr 27. Trends Neurosci. 2014. PMID: 24780507 Free PMC article. Review.
-
Indications for cellular migration from the central nervous system to its draining lymph nodes in CD11c-GFP+ bone-marrow chimeras following EAE.Exp Brain Res. 2017 Jul;235(7):2151-2166. doi: 10.1007/s00221-017-4956-x. Epub 2017 Apr 18. Exp Brain Res. 2017. PMID: 28421248
-
SIV encephalitis lesions are composed of CD163(+) macrophages present in the central nervous system during early SIV infection and SIV-positive macrophages recruited terminally with AIDS.Am J Pathol. 2015 Jun;185(6):1649-65. doi: 10.1016/j.ajpath.2015.01.033. Epub 2015 May 8. Am J Pathol. 2015. PMID: 25963554 Free PMC article.
-
Role of DAMPs and cell death in autoimmune diseases: the example of multiple sclerosis.Genes Immun. 2023 Apr;24(2):57-70. doi: 10.1038/s41435-023-00198-8. Epub 2023 Feb 7. Genes Immun. 2023. PMID: 36750753 Review.
References
-
- McMenamin PG. Distribution and phenotype of dendritic cells and resident tissue macrophages in the dura mater, leptomeninges, and choroid plexus of the rat brain as demonstrated in wholemount preparations. J Comp Neurol. 1999;405:553–562. - PubMed
-
- McMenamin PG, Wealthall RJ, Deverall M, Cooper SJ, Griffin B. Macrophages and dendritic cells in the rat meninges and choroid plexus: three-dimensional localization by environmental scanning electron microscopy and confocal microscopy. Cell Tissue Res. 2003;313:259–269. - PubMed
-
- Kivisäkk P, Mahad DJ, Callahan MK, Sikora K, Trebst C, Tucky B, Wujek J, Ravid R, Staugaitis SM, Lassmann H, Ransohoff RM. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Ann Neurol. 2004;55:627–638. - PubMed
-
- Henkel JS, Engelhardt JI, Siklos L, Simpson EP, Kim SH, Pan T, Goodman JC, Siddique T, Beers DR, Appel SH. Presence of dendritic cells. MCP-1, and activated microglia/macrohages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2003;55:221–235. - PubMed
-
- Fischer HG, Bonifas U, Reichmann G. Phenotype and functions of brain dendritic cells emerging during chronic infection of mice with Toxoplasma gondii. J Immunol. 2000;164:4826–4834. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

