Structural insights into a circadian oscillator
- PMID: 18974343
- PMCID: PMC2588432
- DOI: 10.1126/science.1150451
Structural insights into a circadian oscillator
Abstract
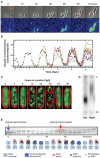
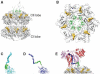
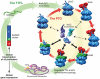
An endogenous circadian system in cyanobacteria exerts pervasive control over cellular processes, including global gene expression. Indeed, the entire chromosome undergoes daily cycles of topological changes and compaction. The biochemical machinery underlying a circadian oscillator can be reconstituted in vitro with just three cyanobacterial proteins, KaiA, KaiB, and KaiC. These proteins interact to promote conformational changes and phosphorylation events that determine the phase of the in vitro oscillation. The high-resolution structures of these proteins suggest a ratcheting mechanism by which the KaiABC oscillator ticks unidirectionally. This posttranslational oscillator may interact with transcriptional and translational feedback loops to generate the emergent circadian behavior in vivo. The conjunction of structural, biophysical, and biochemical approaches to this system reveals molecular mechanisms of biological timekeeping.
Figures
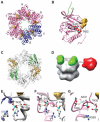
Similar articles
-
The cyanobacterial circadian system: from biophysics to bioevolution.Annu Rev Biophys. 2011;40:143-67. doi: 10.1146/annurev-biophys-042910-155317. Annu Rev Biophys. 2011. PMID: 21332358 Free PMC article. Review.
-
A mathematical model for the Kai-protein-based chemical oscillator and clock gene expression rhythms in cyanobacteria.J Biol Rhythms. 2007 Feb;22(1):69-80. doi: 10.1177/0748730406295749. J Biol Rhythms. 2007. PMID: 17229926
-
The reversible function switching of the circadian clock protein KaiA is encoded in its structure.Biochim Biophys Acta Gen Subj. 2017 Nov;1861(11 Pt A):2535-2542. doi: 10.1016/j.bbagen.2017.08.012. Epub 2017 Aug 24. Biochim Biophys Acta Gen Subj. 2017. PMID: 28844977
-
A cyanobacterial circadian clockwork.Curr Biol. 2008 Sep 9;18(17):R816-R825. doi: 10.1016/j.cub.2008.07.012. Curr Biol. 2008. PMID: 18786387 Free PMC article. Review.
-
Ordered phosphorylation governs oscillation of a three-protein circadian clock.Science. 2007 Nov 2;318(5851):809-12. doi: 10.1126/science.1148596. Epub 2007 Oct 4. Science. 2007. PMID: 17916691 Free PMC article.
Cited by
-
Positioning the Model Bacterial Organelle, the Carboxysome.mBio. 2021 May 11;12(3):e02519-19. doi: 10.1128/mBio.02519-19. mBio. 2021. PMID: 33975941 Free PMC article. Review.
-
Chlamydomonas reinhardtii: duration of its cell cycle and phases at growth rates affected by light intensity.Planta. 2011 Jan;233(1):75-86. doi: 10.1007/s00425-010-1282-y. Epub 2010 Oct 5. Planta. 2011. PMID: 20922544
-
Combined SAXS/EM based models of the S. elongatus post-translational circadian oscillator and its interactions with the output His-kinase SasA.PLoS One. 2011;6(8):e23697. doi: 10.1371/journal.pone.0023697. Epub 2011 Aug 24. PLoS One. 2011. PMID: 21887298 Free PMC article.
-
Complex dynamics of transcription regulation.Biochim Biophys Acta. 2012 Jul;1819(7):657-66. doi: 10.1016/j.bbagrm.2012.03.004. Epub 2012 Mar 28. Biochim Biophys Acta. 2012. PMID: 22484099 Free PMC article. Review.
-
Intermolecular associations determine the dynamics of the circadian KaiABC oscillator.Proc Natl Acad Sci U S A. 2010 Aug 17;107(33):14805-10. doi: 10.1073/pnas.1002119107. Epub 2010 Aug 2. Proc Natl Acad Sci U S A. 2010. PMID: 20679240 Free PMC article.
References
-
- Dunlap JC, Loros JJ, DeCoursey PJ, editors. Chronobiology: Biological Timekeeping. Sinauer; Sunderland, MA: 2004.
-
- Mackey SR, Golden SS. Trends Microbiol. 2007;15:381–388. - PubMed
-
- Johnson CH. In: Methods in Enzymology. Young MW, editor. vol. 393. Academic Press; 2005. pp. 818–837. - PubMed
-
- Mihalcescu I, Hsing W, Leibler S. Nature. 2004;430:81–85. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

