Glia are essential for sensory organ function in C. elegans
- PMID: 18974354
- PMCID: PMC2735448
- DOI: 10.1126/science.1163074
Glia are essential for sensory organ function in C. elegans
Abstract
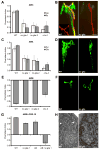
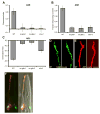
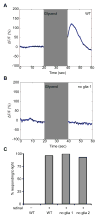
Sensory organs are composed of neurons, which convert environmental stimuli to electrical signals, and glia-like cells, whose functions are not well understood. To decipher glial roles in sensory organs, we ablated the sheath glial cell of the major sensory organ of Caenorhabditis elegans. We found that glia-ablated animals exhibit profound sensory deficits and that glia provide activities that affect neuronal morphology, behavior generation, and neuronal uptake of lipophilic dyes. To understand the molecular bases of these activities, we identified 298 genes whose messenger RNAs are glia-enriched. One gene, fig-1, encodes a labile protein with conserved thrombospondin TSP1 domains. FIG-1 protein functions extracellularly, is essential for neuronal dye uptake, and also affects behavior. Our results suggest that glia are required for multiple aspects of sensory organ function.
Figures

Comment in
-
Neuroscience. A new glance at glia.Science. 2008 Oct 31;322(5902):693-4. doi: 10.1126/science.1166197. Science. 2008. PMID: 18974341 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

