Molecular pharmacology of human Cav3.2 T-type Ca2+ channels: block by antihypertensives, antiarrhythmics, and their analogs
- PMID: 18974361
- PMCID: PMC2682278
- DOI: 10.1124/jpet.108.145672
Molecular pharmacology of human Cav3.2 T-type Ca2+ channels: block by antihypertensives, antiarrhythmics, and their analogs
Abstract
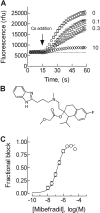
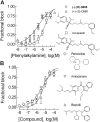
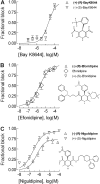
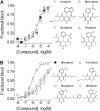
Antihypertensive drugs of the "calcium channel blocker" or "calcium antagonist" class have been used to establish the physiological role of L-type Ca(2+) channels in vascular smooth muscle. In contrast, there has been limited progress on the pharmacology T-type Ca(2+) channels. T-type channels play a role in cardiac pacemaking, aldosterone secretion, and renal hemodynamics, leading to the hypothesis that mixed T- and L-type blockers may have therapeutic advantages over selective L-type blockers. The goal of this study was to identify compounds that block the Ca(v)3.2 T-type channel with high affinity, focusing on two classes of compounds: phenylalkylamines (e.g., mibefradil) and dihydropyridines (e.g., efonidipine). Compounds were tested using a validated Ca(2+) influx assay into a cell line expressing recombinant Ca(v)3.2 channels. This study identified four clinically approved antihypertensive drugs (efonidipine, felodipine, isradipine, and nitrendipine) as potent T-channel blockers (IC(50) < 3 microM). In contrast, other widely prescribed dihydropyridines, such as amlodipine and nifedipine, were 10-fold less potent, making them a more appropriate choice in research studies on the role of L-type currents. In summary, the present results support the notion that many available antihypertensive drugs block a substantial fraction of T-current at therapeutically relevant concentrations, contributing to their mechanism of action.
Figures
Similar articles
-
Actions of mibefradil, efonidipine and nifedipine block of recombinant T- and L-type Ca channels with distinct inhibitory mechanisms.Pharmacology. 2006;78(1):11-20. doi: 10.1159/000094900. Epub 2006 Aug 7. Pharmacology. 2006. PMID: 16899990
-
A selective T-type Ca2+ channel blocker R(-) efonidipine.Naunyn Schmiedebergs Arch Pharmacol. 2008 Jun;377(4-6):411-21. doi: 10.1007/s00210-007-0239-6. Epub 2008 Feb 16. Naunyn Schmiedebergs Arch Pharmacol. 2008. PMID: 18278483
-
Heterogeneity of L- and T-channels in the vasculature: rationale for the efficacy of combined L- and T-blockade.Hypertension. 2009 Apr;53(4):654-60. doi: 10.1161/HYPERTENSIONAHA.108.125831. Epub 2009 Feb 23. Hypertension. 2009. PMID: 19237682
-
Pathophysiological significance of T-type Ca2+ channels: T-type Ca2+ channels and drug development.J Pharmacol Sci. 2005 Nov;99(3):214-20. doi: 10.1254/jphs.fmj05002x5. J Pharmacol Sci. 2005. PMID: 16293935 Review.
-
Efonidipine hydrochloride: a dual blocker of L- and T-type ca(2+) channels.Cardiovasc Drug Rev. 2002 Winter;20(1):81-92. doi: 10.1111/j.1527-3466.2002.tb00084.x. Cardiovasc Drug Rev. 2002. PMID: 12070536 Review.
Cited by
-
In silico identification of T-type calcium channel blockers: A ligand-based pharmacophore mapping approach.J Adv Res. 2016 Nov;7(6):931-944. doi: 10.1016/j.jare.2016.09.004. Epub 2016 Sep 16. J Adv Res. 2016. PMID: 27713840 Free PMC article.
-
Discovery and Development of Calcium Channel Blockers.Front Pharmacol. 2017 May 29;8:286. doi: 10.3389/fphar.2017.00286. eCollection 2017. Front Pharmacol. 2017. PMID: 28611661 Free PMC article. Review.
-
Structural basis for human Cav3.2 inhibition by selective antagonists.Cell Res. 2024 Jun;34(6):440-450. doi: 10.1038/s41422-024-00959-8. Epub 2024 Apr 11. Cell Res. 2024. PMID: 38605177 Free PMC article.
-
1,4-dihydropyridines: the multiple personalities of a blockbuster drug family.Transl Med UniSa. 2012 Oct 11;4:12-26. Print 2012 Sep. Transl Med UniSa. 2012. PMID: 23905059 Free PMC article.
-
Molecular simulations study of novel 1,4-dihydropyridines derivatives with a high selectivity for Cav3.1 calcium channel.Protein Sci. 2015 Nov;24(11):1737-47. doi: 10.1002/pro.2763. Epub 2015 Aug 25. Protein Sci. 2015. PMID: 26256672 Free PMC article.
References
-
- Abernethy DR and Schwartz JB (1999) Drug therapy: calcium-antagonist drugs. N Engl J Med 341 1447-1457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

