Alpha B-crystallin suppresses pressure overload cardiac hypertrophy
- PMID: 18974385
- PMCID: PMC2610480
- DOI: 10.1161/CIRCRESAHA.108.180117
Alpha B-crystallin suppresses pressure overload cardiac hypertrophy
Abstract
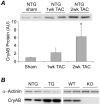
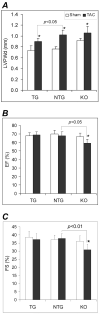
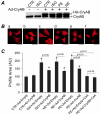
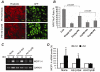
AlphaB-crystallin (CryAB) is the most abundant small heat shock protein (HSP) constitutively expressed in cardiomyocytes. Gain- and loss-of-function studies demonstrated that CryAB can protect against myocardial ischemia/reperfusion injury. However, the role of CryAB or any HSPs in cardiac responses to mechanical overload is unknown. This study addresses this issue. Nontransgenic mice and mice with cardiomyocyte-restricted transgenic overexpression of CryAB or with germ-line ablation of the CryAB/HSPB2 genes were subjected to transverse aortic constriction or sham surgery. Two weeks later, cardiac responses were analyzed by fetal gene expression profiling, cardiac function analyses, and morphometry. Comparison among the 3 sham surgery groups reveals that CryAB overexpression is benign, whereas the knockout is detrimental to the heart as reflected by cardiac hypertrophy and malfunction at 10 weeks of age. Compared to nontransgenic mice, transgenic mouse hearts showed significantly reduced NFAT transactivation and attenuated cardiac hypertrophic responses to transverse aortic constriction but unchanged cardiac function, whereas NFAT transactivation was significantly increased in cardiac and skeletal muscle of the knockout mice at baseline, and they developed cardiac insufficiency at 2 weeks after transverse aortic constriction. CryAB overexpression in cultured neonatal rat cardiomyocytes significantly attenuated adrenergic stimulation-induced NFAT transactivation and hypertrophic growth. We conclude that CryAB suppresses cardiac hypertrophic responses likely through attenuating NFAT signaling and that CryAB and/or HSPB2 are essential for normal cardiac function.
Figures



Comment in
-
Rescuing cardiac malfunction: the roles of the chaperone-like small heat shock proteins.Circ Res. 2008 Dec 5;103(12):1351-3. doi: 10.1161/CIRCRESAHA.108.189720. Circ Res. 2008. PMID: 19059837 Free PMC article. Review. No abstract available.
References
-
- Kumarapeli AR, Wang X. Genetic modification of the heart: chaperones and the cytoskeleton. J Mol Cell Cardiol. 2004;37:1097–1109. - PubMed
-
- Taylor RP, Benjamin IJ. Small heat shock proteins: a new classification scheme in mammals. J Mol Cell Cardiol. 2005;38:433–444. - PubMed
-
- Perng MD, Muchowski PJ, van Den IP, Wu GJ, Hutcheson AM, Clark JI, Quinlan RA. The cardiomyopathy and lens cataract mutation in alpha B-crystallin alters its protein structure, chaperone activity, and interaction with intermediate filaments in vitro. J Biol Chem. 1999;274:33235–33243. - PubMed
-
- Wang X, Robbins J. Heart failure and protein quality control. Circ Res. 2006;99:1315–1328. - PubMed
-
- Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P, Wang Y, Jones WK, Chu G, Kranias EG. Novel cardioprotective role of a small heat-shock protein, Hsp20, against ischemia/reperfusion injury. Circulation. 2005;111:1792–1799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

