Quantitative live imaging of cancer and normal cells treated with Kinesin-5 inhibitors indicates significant differences in phenotypic responses and cell fate
- PMID: 18974392
- PMCID: PMC2597169
- DOI: 10.1158/1535-7163.MCT-08-0684
Quantitative live imaging of cancer and normal cells treated with Kinesin-5 inhibitors indicates significant differences in phenotypic responses and cell fate
Abstract
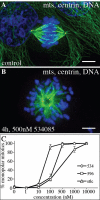
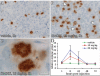
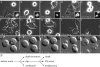
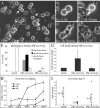
Kinesin-5 inhibitors (K5I) are promising antimitotic cancer drug candidates. They cause prolonged mitotic arrest and death of cancer cells, but their full range of phenotypic effects in different cell types has been unclear. Using time-lapse microscopy of cancer and normal cell lines, we find that a novel K5I causes several different cancer and noncancer cell types to undergo prolonged arrest in monopolar mitosis. Subsequent events, however, differed greatly between cell types. Normal diploid cells mostly slipped from mitosis and arrested in tetraploid G(1), with little cell death. Several cancer cell lines died either during mitotic arrest or following slippage. Contrary to prevailing views, mitotic slippage was not required for death, and the duration of mitotic arrest correlated poorly with the probability of death in most cell lines. We also assayed drug reversibility and long-term responses after transient drug exposure in MCF7 breast cancer cells. Although many cells divided after drug washout during mitosis, this treatment resulted in lower survival compared with washout after spontaneous slippage likely due to chromosome segregation errors in the cells that divided. Our analysis shows that K5Is cause cancer-selective cell killing, provides important kinetic information for understanding clinical responses, and elucidates mechanisms of drug sensitivity versus resistance at the level of phenotype.
Figures

References
-
- Jordan MA, Wilson L. Microtubules as a target for anticancer drugs. Nat Rev Cancer. 2004;4:253–65. - PubMed
-
- Hamel E. Antimitotic natural products and their interactions with tubulin. Med Res Rev. 1996;16:207–31. - PubMed
-
- Rieder CL, Maiato H. Stuck in division or passing through: what happens when cells cannot satisfy the spindle assembly checkpoint. Dev Cell. 2004;7:637–51. - PubMed
-
- Jackson JR, Patrick DR, Dar MM, Huang PS. Targeted anti-mitotic therapies: can we improve on tubulin agents? Nat Rev Cancer. 2007;7:107–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

