Translational strategies exploiting TNF-alpha that sensitize tumors to radiation therapy
- PMID: 18974777
- PMCID: PMC5705082
- DOI: 10.1038/cgt.2008.86
Translational strategies exploiting TNF-alpha that sensitize tumors to radiation therapy
Abstract
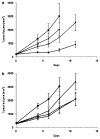
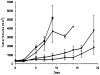
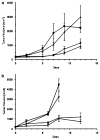
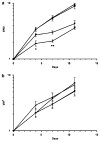
TNFerade is a radioinducible adenoviral vector expressing tumor necrosis factor-alpha (TNF-alpha) (Ad.Egr-TNF) currently in a phase III trial for inoperable pancreatic cancer. We studied B16-F1 melanoma tumors in TNF receptor wild-type (C57BL/6) and deficient (TNFR1,2-/- and TNFR1-/-) mice. Ad.Egr-TNF+IR inhibited tumor growth compared with IR in C57BL/6 but not in receptor-deficient mice. Tumors resistant to TNF-alpha were also sensitive to Ad.Egr-TNF+IR in C57BL/6 mice. Ad.Egr-TNF+IR produced an increase in tumor-associated endothelial cell apoptosis not observed in receptor-deficient animals. Also, B16-F1 tumors in mice with germline deletions of TNFR1,2, TNFR1 or TNF-alpha, or in mice receiving anti-TNF-alpha exhibited radiosensitivity. These results show that tumor-associated endothelium is the principal target for Ad.Egr-TNF radiosensitization and implicate TNF-alpha signaling in tumor radiosensitivity.
Figures

References
-
- Ogawa K, Boucher Y, Kashiwagi S, Fukumura D, Chen D, Gerweck LE. Influence of tumor cell and stroma sensitivity on tumor response to radiation. Cancer Res. 2007;67:4016–4021. - PubMed
-
- Garcia-Barros M, Paris F, Cordon-Cardo C, Lyden D, Rafii S, Haimovitz-Friedman A, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155–1159. - PubMed
-
- Moeller BJ, Dreher MR, Rabbani ZN, Schroeder T, Cao Y, Li CY, et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99–110. - PubMed
-
- Gerweck LE, Vijayappa S, Kurimasa A, Ogawa K, Chen DJ. Tumor cell radiosensitivity is a major determinant of tumor response to radiation. Cancer Res. 2006;66:8352–8355. - PubMed
-
- Suit HD, Willers H. Comment on ‘Tumor response to radiotherapy regulated by endothelial cell apoptosis’ (I) Science. 2003;302:1894. author reply 1894. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

