Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status
- PMID: 18974782
- PMCID: PMC2570490
- DOI: 10.1371/journal.pone.0003577
Magnitude and complexity of rectal mucosa HIV-1-specific CD8+ T-cell responses during chronic infection reflect clinical status
Abstract
Background: The intestinal mucosa displays robust virus replication and pronounced CD4+ T-cell loss during acute human immunodeficiency virus type 1 (HIV-1) infection. The ability of HIV-specific CD8+ T-cells to modulate disease course has prompted intensive study, yet the significance of virus-specific CD8+ T-cells in mucosal sites remains unclear.
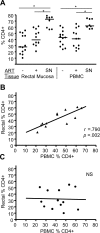
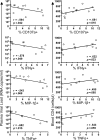
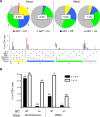
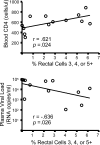
Methods and findings: We evaluated five distinct effector functions of HIVgag-specific CD8+ T-cells in rectal mucosa and blood, individually and in combination, in relationship to clinical status and antiretroviral therapy (ART). In subjects not on ART, the percentage of rectal Gag-specific CD8+ T-cells capable of 3, 4 or 5 simultaneous effector functions was significantly related to blood CD4 count and inversely related to plasma viral load (PVL) (p<0.05). Polyfunctional rectal CD8+ T-cells expressed higher levels of MIP-1beta and CD107a on a per cell basis than mono- or bifunctional cells. The production of TNFalpha, IFN-gamma, and CD107a by Gag-specific rectal CD8+ T-cells each correlated inversely (p<0.05) with PVL, and MIP-1beta expression revealed a similar trend. CD107a and IFN-gamma production were positively related to blood CD4 count (p<0.05), with MIP-1beta showing a similar trend. IL-2 production by rectal CD8+ T-cells was highly variable and generally low, and showed no relationship to viral load or blood CD4 count.
Conclusions: The polyfunctionality of rectal Gag-specific CD8+ T-cells appears to be related to blood CD4 count and inversely related to PVL. The extent to which these associations reflect causality remains to be determined; nevertheless, our data suggest a potentially important role for mucosal T-cells in limiting virus replication during chronic infection.
Conflict of interest statement
Figures



References
-
- Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. - PubMed
-
- Ogg GS, Jin X, Bonhoeffer S, Dunbar PR, Nowak MA, et al. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

