Repetitive N-WASP-binding elements of the enterohemorrhagic Escherichia coli effector EspF(U) synergistically activate actin assembly
- PMID: 18974829
- PMCID: PMC2567903
- DOI: 10.1371/journal.ppat.1000191
Repetitive N-WASP-binding elements of the enterohemorrhagic Escherichia coli effector EspF(U) synergistically activate actin assembly
Abstract
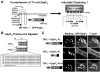
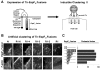
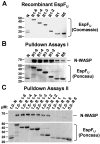
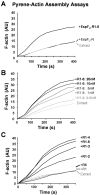
Enterohemorrhagic Escherichia coli (EHEC) generate F-actin-rich adhesion pedestals by delivering effector proteins into mammalian cells. These effectors include the translocated receptor Tir, along with EspF(U), a protein that associates indirectly with Tir and contains multiple peptide repeats that stimulate actin polymerization. In vitro, the EspF(U) repeat region is capable of binding and activating recombinant derivatives of N-WASP, a host actin nucleation-promoting factor. In spite of the identification of these important bacterial and host factors, the underlying mechanisms of how EHEC so potently exploits the native actin assembly machinery have not been clearly defined. Here we show that Tir and EspF(U) are sufficient for actin pedestal formation in cultured cells. Experimental clustering of Tir-EspF(U) fusion proteins indicates that the central role of the cytoplasmic portion of Tir is to promote clustering of the repeat region of EspF(U). Whereas clustering of a single EspF(U) repeat is sufficient to bind N-WASP and generate pedestals on cultured cells, multi-repeat EspF(U) derivatives promote actin assembly more efficiently. Moreover, the EspF(U) repeats activate a protein complex containing N-WASP and the actin-binding protein WIP in a synergistic fashion in vitro, further suggesting that the repeats cooperate to stimulate actin polymerization in vivo. One explanation for repeat synergy is that simultaneous engagement of multiple N-WASP molecules can enhance its ability to interact with the actin nucleating Arp2/3 complex. These findings define the minimal set of bacterial effectors required for pedestal formation and the elements within those effectors that contribute to actin assembly via N-WASP-Arp2/3-mediated signaling pathways.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

