The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair
- PMID: 18974869
- PMCID: PMC2570486
- DOI: 10.1371/journal.pone.0003579
The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair
Abstract
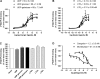
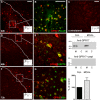
Deciphering the mechanisms regulating the generation of new neurons and new oligodendrocytes, the myelinating cells of the central nervous system, is of paramount importance to address new strategies to replace endogenous damaged cells in the adult brain and foster repair in neurodegenerative diseases. Upon brain injury, the extracellular concentrations of nucleotides and cysteinyl-leukotrienes (cysLTs), two families of endogenous signaling molecules, are markedly increased at the site of damage, suggesting that they may act as "danger signals" to alert responses to tissue damage and start repair. Here we show that, in brain telencephalon, GPR17, a recently deorphanized receptor for both uracil nucleotides and cysLTs (e.g., UDP-glucose and LTD(4)), is normally present on neurons and on a subset of parenchymal quiescent oligodendrocyte precursor cells. We also show that induction of brain injury using an established focal ischemia model in the rodent induces profound spatiotemporal-dependent changes of GPR17. In the lesioned area, we observed an early and transient up-regulation of GPR17 in neurons expressing the cellular stress marker heat shock protein 70. Magnetic Resonance Imaging in living mice showed that the in vivo pharmacological or biotechnological knock down of GPR17 markedly prevents brain infarct evolution, suggesting GPR17 as a mediator of neuronal death at this early ischemic stage. At later times after ischemia, GPR17 immuno-labeling appeared on microglia/macrophages infiltrating the lesioned area to indicate that GPR17 may also acts as a player in the remodeling of brain circuitries by microglia. At this later stage, parenchymal GPR17+ oligodendrocyte progenitors started proliferating in the peri-injured area, suggesting initiation of remyelination. To confirm a specific role for GPR17 in oligodendrocyte differentiation, the in vitro exposure of cortical pre-oligodendrocytes to the GPR17 endogenous ligands UDP-glucose and LTD(4) promoted the expression of myelin basic protein, confirming progression toward mature oligodendrocytes. Thus, GPR17 may act as a "sensor" that is activated upon brain injury on several embryonically distinct cell types, and may play a key role in both inducing neuronal death inside the ischemic core and in orchestrating the local remodeling/repair response. Specifically, we suggest GPR17 as a novel target for therapeutic manipulation to foster repair of demyelinating wounds, the types of lesions that also occur in patients with multiple sclerosis.
Conflict of interest statement
Figures




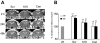
References
-
- Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. - PubMed
-
- Samuelsson B. The discovery of the leukotrienes. Am J Respir Crit Care Med. 2000;161:S2–6. - PubMed
-
- Drazen JM. Leukotrienes in asthma. Adv Exp Med Biol. 2003;525:1–5. - PubMed
-
- Brink C, Dahlen SE, Drazen J, Evans JF, Hay DW, et al. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol Rev. 2003;55:195–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

