AMP-activated protein kinase-regulated activation of the PGC-1alpha promoter in skeletal muscle cells
- PMID: 18974883
- PMCID: PMC2570798
- DOI: 10.1371/journal.pone.0003614
AMP-activated protein kinase-regulated activation of the PGC-1alpha promoter in skeletal muscle cells
Abstract
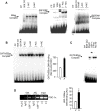
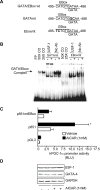
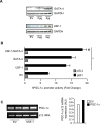
The mechanisms by which PGC-1alpha gene expression is controlled in skeletal muscle remains largely undefined. Thus, we sought to investigate the transcriptional regulation of PGC-1alpha using AICAR, an activator of AMPK, that is known to increase PGC-1alpha expression. A 2.2 kb fragment of the human PGC-1alpha promoter was cloned and sequence analysis revealed that this TATA-less sequence houses putative consensus sites including a GC-box, a CRE, several IRSs, a SRE, binding sites for GATA, MEF2, p 53, NF-kappaB, and EBox binding proteins. AMPK activation for 24 hours increased PGC-1alpha promoter activity with concomitant increases in mRNA expression. The effect of AICAR on transcriptional activation was mediated by an overlapping GATA/EBox binding site at -495 within the PGC-1alpha promoter based on gel shift analyses that revealed increases in GATA/EBox DNA binding. Mutation of the EBox within the GATA/EBox binding site in the promoter reduced basal promoter activity and completely abolished the AICAR effect. Supershift analyses identified USF-1 as a DNA binding transcription factor potentially involved in regulating PGC-1alpha promoter activity, which was confirmed in vivo by ChIP. Overexpression of either GATA-4 or USF-1 alone increased the p851 PGC-1alpha promoter activity by 1.7- and 2.0-fold respectively, while co-expression of GATA-4 and USF-1 led to an additive increase in PGC-1alpha promoter activity. The USF-1-mediated increase in PGC-1alpha promoter activation led to similar increases at the mRNA level. Our data identify a novel AMPK-mediated regulatory pathway that regulates PGC-1alpha gene expression. This could represent a potential therapeutic target to control PGC-1alpha expression in skeletal muscle.
Conflict of interest statement
Figures

 p 2215,
p 2215,  p 1164,
p 1164,  p 851,
p 851,  p501,
p501,  p 191 as shown in Fig. 2.
p 191 as shown in Fig. 2.
References
-
- Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. - PubMed
-
- Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–838. - PubMed
-
- Hawley JA. Exercise as a therapeutic intervention for the prevention and treatment of insulin resistance. Diabetes Metab Res Rev. 2004;20:383–393. - PubMed
-
- Irrcher I, Adhihetty PJ, Joseph AM, Ljubicic V, Hood DA. Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports Med. 2003;33:783–793. - PubMed
-
- Hood DA. Invited Review: contractile activity-induced mitochondrial biogenesis in skeletal muscle. J Appl Physiol. 2001;90:1137–1157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

