HMGB1-dependent triggering of HIV-1 replication and persistence in dendritic cells as a consequence of NK-DC cross-talk
- PMID: 18974890
- PMCID: PMC2571988
- DOI: 10.1371/journal.pone.0003601
HMGB1-dependent triggering of HIV-1 replication and persistence in dendritic cells as a consequence of NK-DC cross-talk
Abstract
Background: HIV-1 has evolved ways to exploit DCs, thereby facilitating viral dissemination and allowing evasion of antiviral immunity. Recently, the fate of DCs has been found to be extremely dependent on the interaction with autologous NK cells, but the mechanisms by which NK-DC interaction controls viral infections remain unclear. Here, we investigate the impact of NK-DC cross-talk on maturation and functions of HIV-infected immature DCs.
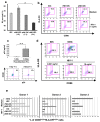
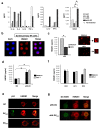
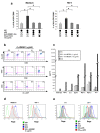
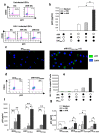
Methodology/principal findings: Immature DCs were derived from primary monocytes, cultured in the presence of IL-4 and GM-CSF. In some experiments, DCs were infected with R5-HIV-1(BaL) or X4-HIV-1(NDK), and viral replication, proviral HIV-DNA and the frequency of infected DCs were measured. Autologous NK cells were sorted and either kept unstimulated in the presence of suboptimal concentration of IL-2, or activated by a combination of PHA and IL-2. The impact of 24 h NK-DC cross-talk on the fate of HIV-1-infected DCs was analyzed. We report that activated NK cells were required for the induction of maturation of DCs, whether uninfected or HIV-1-infected, and this process involved HMGB1. However, the cross-talk between HIV-1-infected DCs and activated NK cells was functionally defective, as demonstrated by the strong impairment of DCs to induce Th1 polarization of naïve CD4 T cells. This was associated with the defective production of IL-12 and IL-18 by infected DCs. Moreover, the crosstalk between activated NK cells and HIV-infected DCs resulted in a dramatic increase in viral replication and proviral DNA expression in DCs. HMGB1, produced both by NK cells and DCs, was found to play a pivotal role in this process, and inhibition of HMGB1 activity by glycyrrhizin, known to bind specifically to HMGB1, or blocking anti-HMGB1 antibodies, abrogated NK-dependent HIV-1 replication in DCs.
Conclusion: These observations provide evidence for the crucial role of NK-DC cross-talk in promoting viral dissemination, and challenge the question of the in vivo involvement of HMGB1 in the triggering of HIV-1 replication and replenishment of viral reservoirs in AIDS.
Conflict of interest statement
Figures

Similar articles
-
HMGB1 Is Involved in IFN-α Production and TRAIL Expression by HIV-1-Exposed Plasmacytoid Dendritic Cells: Impact of the Crosstalk with NK Cells.PLoS Pathog. 2016 Feb 12;12(2):e1005407. doi: 10.1371/journal.ppat.1005407. eCollection 2016 Feb. PLoS Pathog. 2016. PMID: 26871575 Free PMC article.
-
Escape of HIV-1-infected dendritic cells from TRAIL-mediated NK cell cytotoxicity during NK-DC cross-talk--a pivotal role of HMGB1.PLoS Pathog. 2010 Apr 15;6(4):e1000862. doi: 10.1371/journal.ppat.1000862. PLoS Pathog. 2010. PMID: 20419158 Free PMC article.
-
HMGB1, an alarmin promoting HIV dissemination and latency in dendritic cells.Cell Death Differ. 2012 Jan;19(1):96-106. doi: 10.1038/cdd.2011.134. Epub 2011 Oct 28. Cell Death Differ. 2012. PMID: 22033335 Free PMC article. Review.
-
NK/DC crosstalk in anti-viral response.Adv Exp Med Biol. 2012;946:295-308. doi: 10.1007/978-1-4614-0106-3_17. Adv Exp Med Biol. 2012. PMID: 21948375 Review.
-
Natural killer cells, dendritic cells, and the alarmin high-mobility group box 1 protein: a dangerous trio in HIV-1 infection?Curr Opin HIV AIDS. 2011 Sep;6(5):364-72. doi: 10.1097/COH.0b013e328349b089. Curr Opin HIV AIDS. 2011. PMID: 21825870 Review.
Cited by
-
Insufficient natural killer cell responses against retroviruses: how to improve NK cell killing of retrovirus-infected cells.Retrovirology. 2016 Nov 8;13(1):77. doi: 10.1186/s12977-016-0311-8. Retrovirology. 2016. PMID: 27821119 Free PMC article. Review.
-
The Serine Protease CD26/DPP4 in Non-Transformed and Malignant T Cells.Cancers (Basel). 2021 Nov 26;13(23):5947. doi: 10.3390/cancers13235947. Cancers (Basel). 2021. PMID: 34885056 Free PMC article. Review.
-
HMGB1: a double-edged sword and therapeutic target in the female reproductive system.Front Immunol. 2023 Aug 18;14:1238785. doi: 10.3389/fimmu.2023.1238785. eCollection 2023. Front Immunol. 2023. PMID: 37691930 Free PMC article. Review.
-
HMGB1 Is Involved in IFN-α Production and TRAIL Expression by HIV-1-Exposed Plasmacytoid Dendritic Cells: Impact of the Crosstalk with NK Cells.PLoS Pathog. 2016 Feb 12;12(2):e1005407. doi: 10.1371/journal.ppat.1005407. eCollection 2016 Feb. PLoS Pathog. 2016. PMID: 26871575 Free PMC article.
-
An optimized HMGB1 expressed by recombinant rabies virus enhances immunogenicity through activation of dendritic cells in mice.Oncotarget. 2017 Jun 5;8(48):83539-83554. doi: 10.18632/oncotarget.18368. eCollection 2017 Oct 13. Oncotarget. 2017. PMID: 29137362 Free PMC article.
References
-
- Meng G, Wei X, Wu X, Sellers MT, Decker JM, et al. Primary intestinal epithelial cells selectively transfer R5 HIV-1 to CCR5+ cells. Nat Med. 2002;8:150–156. - PubMed
-
- Pope M, Haase AT. Transmission, acute HIV-1 infection and the quest for strategies to prevent infection. Nat Med. 2003;9:847–852. - PubMed
-
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

