Inclusion-body myositis: muscle-fiber molecular pathology and possible pathogenic significance of its similarity to Alzheimer's and Parkinson's disease brains
- PMID: 18974994
- PMCID: PMC2635944
- DOI: 10.1007/s00401-008-0449-0
Inclusion-body myositis: muscle-fiber molecular pathology and possible pathogenic significance of its similarity to Alzheimer's and Parkinson's disease brains
Abstract
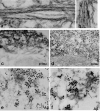
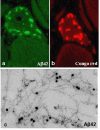
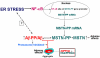
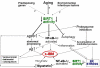
Sporadic inclusion-body myositis (s-IBM), the most common muscle disease of older persons, is of unknown cause and lacks successful treatment. Here we summarize diagnostic criteria and discuss our current understanding of the steps in the pathogenic cascade. While it is agreed that both degeneration and mononuclear-cell inflammation are components of the s-IBM pathology, how each relates to the pathogenesis remains unsettled. We suggest that the intra-muscle-fiber degenerative component plays the primary role, leading to muscle-fiber destruction and clinical weakness, since anti-inflammatory treatments are not of sustained benefit. We discuss possible treatment strategies aimed toward ameliorating a degenerative component, for example, lithium and resveratrol. Also discussed are the intriguing phenotypic similarities between s-IBM muscle fibers and the brains of Alzheimer and Parkinson's diseases, the most common neurodegenerative diseases associated with aging. Similarities include, in the respective tissues, cellular aging, mitochondrial abnormalities, oxidative and endoplasmic-reticulum stresses, proteasome inhibition and multiprotein aggregates.
Figures

Similar articles
-
Sporadic inclusion-body myositis: conformational multifactorial ageing-related degenerative muscle disease associated with proteasomal and lysosomal inhibition, endoplasmic reticulum stress, and accumulation of amyloid-β42 oligomers and phosphorylated tau.Presse Med. 2011 Apr;40(4 Pt 2):e219-35. doi: 10.1016/j.lpm.2010.11.024. Epub 2011 Mar 9. Presse Med. 2011. PMID: 21392932 Review.
-
Sporadic inclusion-body myositis: A degenerative muscle disease associated with aging, impaired muscle protein homeostasis and abnormal mitophagy.Biochim Biophys Acta. 2015 Apr;1852(4):633-43. doi: 10.1016/j.bbadis.2014.09.005. Epub 2014 Sep 18. Biochim Biophys Acta. 2015. PMID: 25241263 Review.
-
Pathogenic considerations in sporadic inclusion-body myositis, a degenerative muscle disease associated with aging and abnormalities of myoproteostasis.J Neuropathol Exp Neurol. 2012 Aug;71(8):680-93. doi: 10.1097/NEN.0b013e31826183c8. J Neuropathol Exp Neurol. 2012. PMID: 22805774 Review.
-
Sporadic inclusion-body myositis and hereditary inclusion-body myopathies: current concepts of diagnosis and pathogenesis.Curr Opin Rheumatol. 1998 Nov;10(6):530-42. doi: 10.1097/00002281-199811000-00005. Curr Opin Rheumatol. 1998. PMID: 9812213 Review.
-
Inclusion-body myositis: a myodegenerative conformational disorder associated with Abeta, protein misfolding, and proteasome inhibition.Neurology. 2006 Jan 24;66(2 Suppl 1):S39-48. doi: 10.1212/01.wnl.0000192128.13875.1e. Neurology. 2006. PMID: 16432144 Review.
Cited by
-
Mitochondrial defects in sporadic inclusion body myositis-causes and consequences.Front Cell Dev Biol. 2024 May 14;12:1403463. doi: 10.3389/fcell.2024.1403463. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38808223 Free PMC article. Review.
-
Oleuropein aglycone protects transgenic C. elegans strains expressing Aβ42 by reducing plaque load and motor deficit.PLoS One. 2013;8(3):e58893. doi: 10.1371/journal.pone.0058893. Epub 2013 Mar 8. PLoS One. 2013. PMID: 23520540 Free PMC article.
-
Muscle biopsy features of idiopathic inflammatory myopathies and differential diagnosis.Auto Immun Highlights. 2014 Sep 10;5(3):77-85. doi: 10.1007/s13317-014-0062-2. eCollection 2014 Dec. Auto Immun Highlights. 2014. PMID: 26000159 Free PMC article. Review.
-
High Fat With High Sucrose Diet Leads to Obesity and Induces Myodegeneration.Front Physiol. 2018 Sep 5;9:1054. doi: 10.3389/fphys.2018.01054. eCollection 2018. Front Physiol. 2018. PMID: 30258366 Free PMC article. Review.
-
Increased plasma amyloid-beta42 protein in sporadic inclusion body myositis.Acta Neuropathol. 2009 Sep;118(3):429-31. doi: 10.1007/s00401-009-0554-8. Epub 2009 Jun 6. Acta Neuropathol. 2009. PMID: 19504113 Free PMC article. No abstract available.
References
-
- Abou-Sleiman PM, Muqit MMK, Wood NW. Expanding insights of mitochondrial dysfunction in Parkinson's disease. Nature Rev Neurosci. 2006;7:207–219. - PubMed
-
- Askanas V, Alvarez RB, Engel WK. β-amyloid precursor epitopes in muscle fibers of inclusion body myositis. Ann Neurol. 1993;34:551–560. - PubMed
-
- Askanas V, Alvarez RB, Mirabella M, Engel WK. Use of antineurofilament antibody to identify paired-helical filaments in inclusion-body myositis. Ann Neurol. 1996;39:389–391. - PubMed
-
- Askanas V, Engel WK, Alvarez RB, McFerrin J, Broccolini A. Novel immunolocalization of α-synuclein in human muscle of inclusion-body myositis, regenerating and necrotic muscle fibers, and at neuromuscular junctions. J Neuropathol Exp Neurol. 2000;59:592–598. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

