A phase I study of combination therapy with S-1 and irinotecan (CPT-11) in patients with advanced colorectal cancer
- PMID: 18974999
- PMCID: PMC12160135
- DOI: 10.1007/s00432-008-0480-5
A phase I study of combination therapy with S-1 and irinotecan (CPT-11) in patients with advanced colorectal cancer
Abstract
Purpose: The aim of this study was to determine the maximum tolerated dose, recommended dose and dose-limiting toxicities of irinotecan (CPT-11) plus S-1 in advanced colorectal cancer.
Methods: S-1 was administered orally at 80 mg/m(2) per day for 14 consecutive days followed by a 2-week rest. CPT-11 was given intravenously on days 1 and 15 of each course, at an initial dose of 80 mg/m(2) per day, stepping up to 100, 120 or 150 mg/m(2) per day. Courses were repeated every 4 weeks, unless disease progression or severe toxicities were observed.
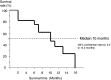
Results: A total of 21 patients were entered in this study. The maximum tolerated dose of CPT-11 was considered to be 150 mg/m(2), because 2 of 3 patients developed dose-limiting toxicities such as leukopenia, neutropenia, diarrhea and anorexia. The recommend dose of CPT-11 was set at 120 mg/m(2). Tumor response rate was 42.8% and median progression-free survival time was 10 months (95% confidential interval, 6.0-14.0 months).
Conclusion: A combination of S-1 and CPT-11 showed a good safety profile and can be recommended for further phase II studies in patients with colorectal cancer.
Similar articles
-
The use of irinotecan, oxaliplatin and raltitrexed for the treatment of advanced colorectal cancer: systematic review and economic evaluation.Health Technol Assess. 2008 May;12(15):iii-ix, xi-162. doi: 10.3310/hta12150. Health Technol Assess. 2008. PMID: 18462574
-
FOLFIRINOX-3 plus bevacizumab (bFOLFIRINOX3) in chemo-refractory metastatic colorectal cancer: a multicenter phase II trial.Future Oncol. 2025 Mar;21(6):699-706. doi: 10.1080/14796694.2025.2461446. Epub 2025 Feb 6. Future Oncol. 2025. PMID: 39913183 Free PMC article. Clinical Trial.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Irinotecan chemotherapy combined with fluoropyrimidines versus irinotecan alone for overall survival and progression-free survival in patients with advanced and/or metastatic colorectal cancer.Cochrane Database Syst Rev. 2016 Feb 12;2(2):CD008593. doi: 10.1002/14651858.CD008593.pub3. Cochrane Database Syst Rev. 2016. PMID: 26869023 Free PMC article.
-
Phase I study of S-1 combined with irinotecan (CPT-11) in patients with advanced colorectal cancer.Oncology. 2007;72(1-2):58-63. doi: 10.1159/000111095. Epub 2007 Nov 13. Oncology. 2007. PMID: 17998791 Clinical Trial.
Cited by
-
Oral maintenance therapy using apatinib combined with S-1/capecitabine for esophageal squamous cell carcinoma with residual disease after definitive chemoradiotherapy.Aging (Albany NY). 2021 Mar 10;13(6):8408-8420. doi: 10.18632/aging.202652. Epub 2021 Mar 10. Aging (Albany NY). 2021. PMID: 33713398 Free PMC article.
-
REDOMA: Bayesian random-effects dose-optimization meta-analysis using spike-and-slab priors.Stat Med. 2024 Aug 15;43(18):3484-3502. doi: 10.1002/sim.10107. Epub 2024 Jun 10. Stat Med. 2024. PMID: 38857904 Free PMC article.
-
Phase II study of irinotecan/S-1 combination chemotherapy for patients with oxaliplatin-refractory colorectal cancer.Invest New Drugs. 2011 Oct;29(5):1050-6. doi: 10.1007/s10637-010-9409-3. Epub 2010 Mar 19. Invest New Drugs. 2011. PMID: 20238142 Clinical Trial.
-
Current Development of Anti-Cancer Drug S-1.J Clin Diagn Res. 2016 Nov;10(11):XE01-XE05. doi: 10.7860/JCDR/2016/19345.8776. Epub 2016 Nov 1. J Clin Diagn Res. 2016. PMID: 28050491 Free PMC article. Review.
References
-
- Bajetta E, Di Bartolomeo M, Mariani L, Cassata A, Artale S, Frustaci S et al (2003) Randomized multicenter phase II trial of two different schedules of irinotecan combined with capecitabine as first-line treatment in metastatic colorectal carcinoma. Cancer 100:279–287. doi:10.1002/cncr.11910 - PubMed
-
- Bajetta E, Di Bartolomeo M, Buzzoni R, Mariani L, Zilembo N, Ferrario E et al (2007) Uracil/ftorafur/leucovorin combined with irinotecan (TEGAFIRI) or oxaliplatin (TEGAFOX) as first-line treatment for metastatic colorectal cancer patients: results of randomized phase II study. Br J Cancer 96:439–444. doi:10.1038/sj.bjc.6603493 - PMC - PubMed
-
- Borner MM, Bernhard J, Dietrich D, Popescu R, Wernli M, Saletti P et al (2005) A randomized phase II trial of capecitabine and two different schedules of irinotecan in first-line treatment of metastatic colorectal cancer: efficacy, quality-of-life and toxicity. Ann Oncol 16:282–288. doi:10.1093/annonc/mdi047 - PubMed
-
- Colucci G, Gebbia V, Paoletti G, Giuliani F, Caruso M, Gebbia N et al (2005) Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: multicenter study of the Gruppo Oncologico Dell’italia Meridionale. J Clin Oncol 23:4866–4875. doi:10.1200/JCO.2005.07.113 - PubMed
-
- Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P et al (2000) Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomized trial. Lancet 355:1041–1047. doi:10.1016/S0140-6736(00)02034-1 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical