Cytochrome c oxidase: exciting progress and remaining mysteries
- PMID: 18975062
- PMCID: PMC4012550
- DOI: 10.1007/s10863-008-9181-7
Cytochrome c oxidase: exciting progress and remaining mysteries
Abstract
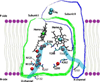
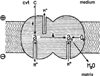
Cytochrome c oxidase generates a proton motive force by two separate mechanisms. The first mechanism is similar to that postulated by Peter Mitchell, and is based on electrons and protons used to generate water coming from opposite sides of the membrane. The second mechanism was not initially anticipated, but is now firmly established as a proton pump. A brief review of the current state of our understanding of the proton pump of cytochrome oxidase is presented. We have come a long way since the initial observation of the pump by Mårten Wikström in 1977, but a number of essential questions remain to be answered.
Figures

References
-
- Abramson J, Riistama S, et al. The structure of the ubiquinol oxidase from Escherichia coli and its ubiquinone binding site. Nature Struct Biol. 2000;7:910–917. - PubMed
-
- Artzatbanov VY, Konstantinov AA, et al. Involvement of intramitochondrial protons in redox reactions of cytochrome a. FEBS Lett. 1978;87:180–185. - PubMed
-
- Babcock GT, Varotsis C. Discrete steps in dioxygen activation—the cytochrome oxidase/O2 reaction. J Bioenerg Biomemb. 1993;25(2):71–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

