Prior authorization for biologic disease-modifying antirheumatic drugs: a description of US Medicaid programs
- PMID: 18975371
- PMCID: PMC3099624
- DOI: 10.1002/art.24191
Prior authorization for biologic disease-modifying antirheumatic drugs: a description of US Medicaid programs
Abstract
Objective: To evaluate state Medicaid prior authorization programs for biologic disease-modifying antirheumatic drugs (DMARDs).
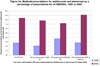
Methods: We obtained biologic DMARD prior authorization policy information from state Medicaid programs. Using aggregate Medicaid drug spending data, we calculated the proportion of DMARD prescriptions and spending attributed to adalimumab and etanercept in 1999 and 2005 and compared the changes in these proportions in states with and without prior authorization policies. Infliximab and other infused DMARDs were not included because of substantial missing data.
Results: Thirty-two states required prior authorization for > or = 1 biologic DMARD, with wide variation in the specific agents covered and the criteria required for a drug to be authorized. There were 18 states with prior authorization requirements for adalimumab or etanercept. States that implemented prior authorization for these agents initially had lower use of the targeted medications, but use increased over time to a level similar to that in states that did not have prior authorization requirements.
Conclusion: States vary widely in their implementation of prior authorization policies to limit use of biologic DMARDs. Although it appears that these policies may have a short-term effect on the use of targeted medications, this effect does not appear to be sustained. The clinical impact and appropriateness of such policies is not clear from our data and should be studied further.
Figures




References
-
- Bruen B, Ghosh A. Medicaid prescription drug spending and use. Washington, DC: Kaiser Commission on Medicaid and the Uninsured; 2004. Jun, 2004.
-
- National Pharmaceutical Council. Pharmaceutical benefits under state medical assistance programs, 2005–2006. Reston, VA: 2006.
-
- Mello MM, Studdert DM, Brennan TA. The pharmaceutical industry versus Medicaid - limits of state initiatives to control prescription-drug costs. N Engl J Med. 2004;350:608–613. - PubMed
-
- Fischer MA, Cheng HC, Schneeweiss S, Avorn J, Solomon DH. Prior authorization policies for selective cyclooxygenase-2 inhibitors in Medicaid: a policy review. Medical Care. 2006;44:658–663. - PubMed
-
- Fischer MA, Choudhry NK, Winkelmayer WC. Impact of Medicaid prior authorization on angiotensin receptor blockers: Can policy promote rational prescribing? Health Affairs. 2007;26:800–807. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

