Disruption of murine cardiac allograft acceptance by latent cytomegalovirus
- PMID: 18976295
- PMCID: PMC2626146
- DOI: 10.1111/j.1600-6143.2008.02457.x
Disruption of murine cardiac allograft acceptance by latent cytomegalovirus
Abstract
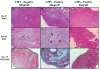
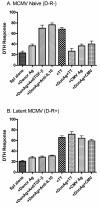
Cytomegalovirus (CMV) reactivation is a well-described complication of solid organ transplantation. These studies were performed to (1) determine if cardiac allograft transplantation of latently infected recipients results in reactivation of CMV and (2) determine what impact CMV might have on development of graft acceptance/tolerance. BALB/c cardiac allografts were transplanted into C57BL/6 mice with/without latent murine CMV (MCMV). Recipients were treated with gallium nitrate induction and monitored for graft survival, viral immunity and donor reactive DTH responses. Latently infected allograft recipients had approximately 80% graft loss by 100 days after transplant, compared with approximately 8% graft loss in naïve recipients. PCR evaluation demonstrated that MCMV was transmitted to cardiac grafts in all latently infected recipients, and 4/8 allografts had active viral transcription compared to 0/6 isografts. Latently infected allograft recipients showed intragraft IFN-alpha expression consistent with MCMV reactivation, but MCMV did not appear to negatively influence regulatory gene expression. Infected allograft recipients had disruption of splenocyte DTH regulation, but recipient splenocytes remained unresponsive to donor antigen even after allograft losses. These data suggest that transplantation in an environment of latent CMV infection may reactivate virus, and that intragraft responses disrupt development of allograft acceptance.
Figures




Similar articles
-
Cytomegalovirus chemokine receptor M33 knockout reduces chronic allograft rejection in a murine aortic transplant model.Transpl Immunol. 2021 Feb;64:101359. doi: 10.1016/j.trim.2020.101359. Epub 2020 Dec 7. Transpl Immunol. 2021. PMID: 33301898
-
Allogeneic stimulation causes transcriptional reactivation of latent murine cytomegalovirus.Transplant Proc. 2009 Jun;41(5):1927-31. doi: 10.1016/j.transproceed.2009.02.086. Transplant Proc. 2009. PMID: 19545758 Free PMC article.
-
Patterns of allosensitization in allograft recipients: long-term cardiac allograft acceptance is associated with active alloantibody production in conjunction with active inhibition of alloreactive delayed-type hypersensitivity.Transplantation. 1998 Apr 27;65(8):1115-23. doi: 10.1097/00007890-199804270-00017. Transplantation. 1998. PMID: 9583874
-
A model for reactivation of CMV from latency.J Clin Virol. 2002 Aug;25 Suppl 2:S123-36. doi: 10.1016/s1386-6532(02)00088-4. J Clin Virol. 2002. PMID: 12361763 Review.
-
Cytomegalovirus infection and cardiac allograft vasculopathy.Transpl Infect Dis. 1999 Jun;1(2):115-26. doi: 10.1034/j.1399-3062.1999.010205.x. Transpl Infect Dis. 1999. PMID: 11428979 Review.
Cited by
-
Occult cytomegalovirus in vivarium-housed mice may influence transplant allograft acceptance.Transpl Immunol. 2010 May;23(1-2):86-91. doi: 10.1016/j.trim.2010.03.005. Epub 2010 Mar 20. Transpl Immunol. 2010. PMID: 20307665 Free PMC article.
-
Antiviral prevention of sepsis induced cytomegalovirus reactivation in immunocompetent mice.Antiviral Res. 2010 Mar;85(3):496-503. doi: 10.1016/j.antiviral.2009.12.004. Epub 2009 Dec 11. Antiviral Res. 2010. PMID: 20004216 Free PMC article.
-
Early acceptance of renal allografts in mice is dependent on foxp3(+) cells.Am J Pathol. 2011 Apr;178(4):1635-45. doi: 10.1016/j.ajpath.2010.12.024. Am J Pathol. 2011. PMID: 21435448 Free PMC article.
-
Don't Go Breaking My Heart: MCMV as a Model for HCMV-Associated Cardiovascular Diseases.Pathogens. 2021 May 18;10(5):619. doi: 10.3390/pathogens10050619. Pathogens. 2021. PMID: 34069957 Free PMC article. Review.
-
Trichinella spiralis infection changes immune response in mice performed abdominal heterotopic cardiac transplantation and prolongs cardiac allograft survival time.Parasitol Res. 2016 Jan;115(1):407-14. doi: 10.1007/s00436-015-4762-y. Epub 2015 Oct 19. Parasitol Res. 2016. PMID: 26481486
References
-
- Orosz CG, Wakely E, Sedmak DD, Bergese SD, VanBuskirk AM. Prolonged murine cardiac allograft acceptance: characteristics of persistent active alloimmunity after treatment with gallium nitrate versus anti-CD4 monoclonal antibody. Transplantation. 1997;63(8):1109–1117. - PubMed
-
- VanBuskirk AM, Wakely ME, Sirak JH, Orosz CG. Patterns of allosensitization in allograft recipients: long-term cardiac allograft acceptance is associated with active alloantibody production in conjunction with active inhibition of alloreactive delayed-type hypersensitivity. Transplantation. 1998;65(8):1115–1123. - PubMed
-
- Orosz CG, Wakely E, Bergese SD, VanBuskirk AM, Ferguson RM, Mullet D, et al. Prevention of Murine Cardiac Allograft Rejection with Gallium Nitrate: Comparison with Anti-CD4 Monoclonal Antibody 1. Transplantation. 1996;61(5):783–791. - PubMed
-
- Bickerstaff AA, VanBuskirk AM, Wakely E, Orosz CG. Transforming growth factor-beta and interleukin-10 subvert alloreactive delayed type hypersensitivity in cardiac allograft acceptor mice. Transplantation. 2000;69(7):1517–1520. - PubMed
-
- Bickerstaff AA, Xia D, Pelletier RP, Orosz CG. Mechanisms of graft acceptance: evidence that plasminogen activator controls donor-reactive delayed-type hypersensitivity responses in cardiac allograft acceptor mice. J Immunol. 2000;164(10):5132–5139. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

