Graft and patient survival in kidney transplant recipients selected for de novo steroid-free maintenance immunosuppression
- PMID: 18976304
- PMCID: PMC2626128
- DOI: 10.1111/j.1600-6143.2008.02442.x
Graft and patient survival in kidney transplant recipients selected for de novo steroid-free maintenance immunosuppression
Abstract
Steroid-free regimen is increasingly employed in kidney transplant recipients across transplant centers. However, concern remains because of the unknown impact of such an approach on long-term graft and patient survival. We studied the outcomes of steroid-free immunosuppression in a population-based U.S. cohort of kidney transplant recipients. All adult solitary kidney transplant recipients engrafted between January 1, 2000 and December 31, 2006 were stratified according to whether they were selected for a steroid-free or steroid-containing regimen at discharge. Multivariate Cox regression models were used to estimate graft and patient survival. The impact of the practice pattern on steroid use at individual transplant centers was analyzed. Among 95 755 kidney transplant recipients, 17.2% were steroid-free at discharge (n = 16 491). Selection for a steroid-free regimen was associated with reduced risks for graft failure and death at 1 year (HR 0.78, 95% CI 0.72-0.85, and HR 0.73, 95% CI 0.65-0.82, respectively, p < 0.0001) and 4 years (HR 0.83, 95% CI 0.78-0.87, and HR 0.76, 95% CI 0.71-0.83, respectively, p < 0.0001). This association was mostly observed at individual centers where less than 65% of recipients were discharged on the steroid-containing regimen. De novo steroid-free immunosuppression as currently practiced in the United States appears to carry no increased risk of adverse clinical outcomes in the intermediate term.
Figures
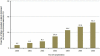


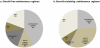
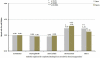
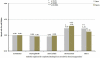
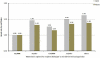
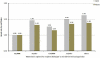
Comment in
-
Steroid withdrawal: moving on to the next questions.Am J Transplant. 2009 Jan;9(1):3-4. doi: 10.1111/j.1600-6143.2008.02465.x. Epub 2008 Nov 27. Am J Transplant. 2009. PMID: 19067669 No abstract available.
Similar articles
-
Graft survival of pediatric kidney transplant recipients selected for de novo steroid avoidance-a propensity score-matched study.Nephrol Dial Transplant. 2017 Aug 1;32(8):1424-1431. doi: 10.1093/ndt/gfx193. Nephrol Dial Transplant. 2017. PMID: 28810723 Free PMC article.
-
Influence of induction modality on the outcome of deceased donor kidney transplant recipients discharged on steroid-free maintenance immunosuppression.Transplantation. 2012 Apr 27;93(8):799-805. doi: 10.1097/TP.0b013e3182472898. Transplantation. 2012. PMID: 22290269
-
Assessing Long-Term Adverse Outcomes in Older Kidney Transplant Recipients: A Propensity Score-Matched Comparison of Early Steroid Withdrawal Versus Continuous Steroid Immunosuppression Using a Large Real-World Database.Drugs Aging. 2024 Nov;41(11):915-927. doi: 10.1007/s40266-024-01147-4. Epub 2024 Oct 17. Drugs Aging. 2024. PMID: 39417973
-
Calcineurin inhibitor-free immunosuppression in pediatric renal transplantation: a viable option?Paediatr Drugs. 2011 Feb 1;13(1):49-69. doi: 10.2165/11538530-000000000-00000. Paediatr Drugs. 2011. PMID: 21162600 Review.
-
Steroid-free maintenance immunosuppression in kidney transplantation: is it time to consider it as a standard therapy?Kidney Int. 2009 Oct;76(8):825-30. doi: 10.1038/ki.2009.248. Epub 2009 Jul 22. Kidney Int. 2009. PMID: 19625995 Free PMC article. Review.
Cited by
-
Early steroid withdrawal and kidney transplant outcomes in first-transplant and retransplant recipients.Nephrol Dial Transplant. 2025 Apr 1;40(4):662-670. doi: 10.1093/ndt/gfae218. Nephrol Dial Transplant. 2025. PMID: 39349991
-
National Variation in Use of Immunosuppression for Kidney Transplantation: A Call for Evidence-Based Regimen Selection.Am J Transplant. 2016 Aug;16(8):2453-62. doi: 10.1111/ajt.13758. Epub 2016 Mar 31. Am J Transplant. 2016. PMID: 26901466 Free PMC article.
-
Role of steroid maintenance in sensitized kidney transplant recipients.World J Transplant. 2015 Sep 24;5(3):102-9. doi: 10.5500/wjt.v5.i3.102. World J Transplant. 2015. PMID: 26421263 Free PMC article.
-
Graft survival of pediatric kidney transplant recipients selected for de novo steroid avoidance-a propensity score-matched study.Nephrol Dial Transplant. 2017 Aug 1;32(8):1424-1431. doi: 10.1093/ndt/gfx193. Nephrol Dial Transplant. 2017. PMID: 28810723 Free PMC article.
-
Ten-year outcome after rapid discontinuation of prednisone in adult primary kidney transplantation.Clin J Am Soc Nephrol. 2012 Mar;7(3):494-503. doi: 10.2215/CJN.08630811. Epub 2012 Jan 26. Clin J Am Soc Nephrol. 2012. PMID: 22282482 Free PMC article.
References
-
- Murray JE, et al. Prolonged survival of human-kidney homografts by immunosuppressive drug therapy. N Engl J Med. 1963;268:1315–23. - PubMed
-
- Reemtsma K, et al. Reversal of Early Graft Rejection after Renal Heterotransplantation in Man. JAMA. 1964;187:691–6. - PubMed
-
- Bell PR, et al. Reversal of acute clinical and experimental organ rejection using large doses of intravenous prednisolone. Lancet. 1971;1(7705):876–80. - PubMed
-
- Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant. 2002;2(9):807–18. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

