Systematic review of dexketoprofen in acute and chronic pain
- PMID: 18976451
- PMCID: PMC2585070
- DOI: 10.1186/1472-6904-8-11
Systematic review of dexketoprofen in acute and chronic pain
Abstract
Background: Dexketoprofen, an NSAID used in the management of acute and chronic pains, is licensed in several countries but has not previously been the subjected of a systematic review. We used published and unpublished information from randomised clinical trials (RCTs) of dexketoprofen in painful conditions to assess evidence on efficacy and harm.
Methods: PubMed and Cochrane Central were searched for RCTs of dexketoprofen for pain of any aetiology. Reference lists of retrieved articles and reviews were also searched. Menarini Group produced copies of published and unpublished studies (clinical trial reports). Data were abstracted into a standard form. For studies reporting results of single dose administration, the number of patients with at least 50% pain relief was derived and used to calculate the relative benefit (RB) and number-needed-to-treat (NNT) for one patient to achieve at least 50% pain relief compared with placebo.
Results: Thirty-five trials were found in acute pain and chronic pain; 6,380 patients were included, 3,381 receiving dexketoprofen. Information from 16 trials (almost half the total patients) was obtained from clinical trial reports from previously unpublished trials or abstracts. Almost all of the trials were of short duration in acute conditions or recent onset pain.All 12 randomised trials that compared dexketoprofen (any dose) with placebo found dexketoprofen to be statistically superior. Five trials in postoperative pain yielded NNTs for 12.5 mg dexketoprofen of 3.5 (2.7 to 4.9), 25 mg dexketoprofen of 3.0 (2.4 to 3.9), and 50 mg dexketoprofen of 2.1 (1.5 to 3.5). In 29/30 active comparator trials, dexketoprofen at the dose used was at least equivalent in efficacy to comparator drugs. Adverse event withdrawal rates were low in postoperative pain and somewhat higher in trials of longer duration; no serious adverse events were reported.
Conclusion: Dexketoprofen was at least as effective as other NSAIDs and paracetamol/opioid combinations. While adverse event withdrawal was not different between dexketoprofen and comparator analgesics, the different conditions and comparators studies precluded any formal analysis. Exposure was limited, and no conclusions could be drawn about safety in terms of serious adverse events like gastrointestinal bleeding or cardiovascular events.
Figures
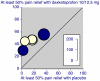
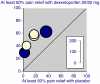
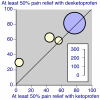
References
-
- Barbanoj MJ, Gich I, Artigas R, Tost D, Moros C, Antonijoan RM, García ML, Mauleon D. Pharmacokinetics of dexketoprofen trometamol in healthy volunteers after single and repeated oral doses. Drugs. 1998;52:24–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

