The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain
- PMID: 18976692
- PMCID: PMC2701510
- DOI: 10.1016/j.neuroscience.2008.10.004
The contributing role of CD14 in toll-like receptor 4 dependent neuropathic pain
Abstract
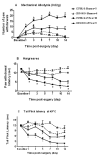
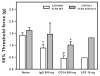
We have previously demonstrated that CNS toll-like receptor 4 (TLR4) plays a key role in the development of behavioral hypersensitivity in a rodent model of neuropathic pain, spinal nerve L5 transection (L5Tx). TLR4 is a well-known receptor for lipopolysaccharide (LPS) in innate immune responses. In the current study, we further investigated the role of CD14, an accessory molecule in the LPS-TLR4 signaling pathway, in the development of L5Tx-induced neuropathic pain. CD14 knockout (KO) mice displayed significantly decreased behavioral sensitivity (mechanical allodynia and thermal hyperalgesia) as early as day 1 post-L5Tx, indicating a nociceptive role of CD14. By flow cytometric analyses, we observed significantly elevated microglial surface CD14 expression in the ipsilateral lumbar spinal cord 3 days post-L5Tx, as well as remarkable increases in microglial size (via forward scatter (FSC)) and granularity (via side scatter (SSC)). Further, intrathecal injection of soluble CD14 induced significantly greater mechanical hypersensitivity in wild type (C3H/HeN) mice compared with TLR4-deficient (C3H/HeJ) mice. Together, these data demonstrate that CD14 plays a contributing role in TLR4-dependent nerve injury-induced neuropathic pain.
Figures






References
-
- Aravalli RN, Peterson PK, Lokensgard JR. Toll-like receptors in defense and damage of the central nervous system. J Neuroimmune Pharmacol. 2007;2:297–312. - PubMed
-
- Bas S, Gauthier BR, Spenato U, Stingelin S, Gabay C. CD14 Is an Acute-Phase Protein. J Immunol. 2004;172:4470–4479. - PubMed
-
- Cauwels A, Frei K, Sansano S, Fearns C, Ulevitch R, Zimmerli W, Landmann R. The Origin and Function of Soluble CD14 in Experimental Bacterial Meningitis. J Immunol. 1999a;162:4762–4772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

