Microsporidia evolved from ancestral sexual fungi
- PMID: 18976912
- PMCID: PMC2654606
- DOI: 10.1016/j.cub.2008.09.030
Microsporidia evolved from ancestral sexual fungi
Abstract
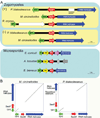
Microsporidia are obligate, intracellular eukaryotic pathogens that infect animal cells, including humans [1]. Previous studies suggested microsporidia share a common ancestor with fungi [2-7]. However, the exact nature of this phylogenetic relationship is unclear because of unusual features of microsporidial genomes, which are compact with fewer and highly divergent genes [8]. As a consequence, it is unclear whether microsporidia evolved from a specific fungal lineage, or whether microsporidia are a sister group to all fungi. Here, we present evidence addressing this controversial question that is independent of sequence-based phylogenetic reconstruction, but rather based on genome structure. In the zygomycete basal fungal lineage, the sex locus is a syntenic gene cluster governing sexual reproduction in which a high mobility group (HMG) transcription-factor gene is flanked by triose-phosphate transporter (TPT) and RNA helicase genes [9]. Strikingly, microsporidian genomes harbor a sex-related locus with the same genes in the same order. Genome-wide synteny analysis reveals multiple other loci conserved between microsporidia and zygomycetes to the exclusion of all other fungal lineages with sequenced genomes. These findings support the hypothesis that microsporidia are true fungi that descended from a zygomycete ancestor and suggest microsporidia may have an extant sexual cycle.
Figures

References
-
- Keeling PJ, Fast NM. Microsporidia: Biology and evolution of highly reduced intracellular parasites. Annu. Rev. Microbiol. 2002;56:93. - PubMed
-
- Edlind TD, Li J, Visvesvara GS, Vodkin MH, McLaughlin GL, Katiyar SK. Phylogenetic analysis of beta-tubulin sequences from amitochondrial protozoa. Mol. Phylogenet. Evol. 1996;5:359–367. - PubMed
-
- Keeling PJ, Doolittle WF. Alpha-tubulin from early-diverging eukaryotic lineages and the evolution of the tubulin family. Mol. Biol. Evol. 1996;13:1297–1305. - PubMed
-
- Keeling PJ. Congruent evidence from alpha-tubulin and beta-tubulin gene phylogenies for a zygomycete origin of microsporidia. Fungal Genet. Biol. 2003;38:298–309. - PubMed
-
- James TY, Kauff F, Schoch CL, Matheny PB, Hofstetter V, Cox CJ, Celio G, Gueidan C, Fraker E, Miadlikowska J, et al. Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature. 2006;443:818–822. - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

