AHR signaling in prostate growth, morphogenesis, and disease
- PMID: 18977204
- PMCID: PMC2918267
- DOI: 10.1016/j.bcp.2008.09.039
AHR signaling in prostate growth, morphogenesis, and disease
Abstract
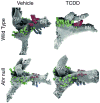
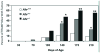
Most evidence of aryl hydrocarbon receptor (AHR) signaling in prostate growth, morphogenesis, and disease stems from research using 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) to pharmacologically activate the AHR at various stages of development. This review discusses effects of TCDD on prostate morphogenesis and highlights interactions between AHR and other signaling pathways during normal and aberrant prostate growth. Although AHR signaling modulates estrogen and androgen signaling in other tissues, crosstalk between these steroid hormone receptors and AHR signaling cannot account for actions of TCDD on prostate morphogenesis. Instead, the AHR appears to act within a cooperative framework of developmental signals to regulate timing and patterning of prostate growth. Inappropriate activation of AHR signaling as a result of early life TCDD exposure disrupts the balance of these signals, impairs prostate morphogenesis, and has an imprinting effect on the developing prostate that predisposes to prostate disease in adulthood. Mechanisms of AHR signaling in prostate growth and disease are only beginning to be unraveled and recent studies have revealed its interactions with WNT5A, retinoic acid, fibroblast growth factor 10, and vascular endothelial growth factor signaling pathways.
Figures


Comment in
-
Note to readers. Re: "AHR signaling in prostate growth, morphogenesis, and disease".Biochem Pharmacol. 2009 Jul 1;78(1):104. doi: 10.1016/j.bcp.2009.03.030. Epub 2009 Apr 5. Biochem Pharmacol. 2009. PMID: 19447229 No abstract available.
References
-
- Cunha GR, Chung LW. Stromal-epithelial interactions--I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem. 1981;14:1317–24. - PubMed
-
- Cunha GR, Lung B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool. 1978;205:181–93. - PubMed
-
- Lin TM, Rasmussen NT, Moore RW, Albrecht RM, Peterson RE. Region-specific inhibition of prostatic epithelial bud formation in the urogenital sinus of C57BL/6 mice exposed in utero to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2003;76:171–81. - PubMed
-
- Timms BG, Mohs TJ, Didio LJ. Ductal budding and branching patterns in the developing prostate. J Urol. 1994;151:1427–32. - PubMed
-
- Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34:961–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

