In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses
- PMID: 18977404
- PMCID: PMC2813682
- DOI: 10.1016/j.vaccine.2008.09.092
In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses
Abstract
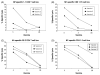
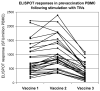
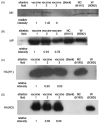
We evaluated three commercial trivalent inactivated vaccines (TIVs) from the 2007-2008 season in terms of their ability to elicit in vitro T cell responses. T cell-mediated immunity may offer a more cross-reactive vaccine approach for the prevention of pandemic or epidemic influenza. Human cytotoxic T cell lines demonstrated differences in matrix protein 1 and nucleocapsid protein recognition of autologous target cells. Peripheral blood mononuclear cells stimulated with each of the TIVs showed statistically significant differences between the vaccines in the numbers of IFNgamma producing cells activated. These data suggest that TIV vaccines are not similar in their ability to activate human T cell responses.
Figures

Similar articles
-
Influenza A virus matrix protein 1-specific human CD8+ T-cell response induced in trivalent inactivated vaccine recipients.J Virol. 2008 Sep;82(18):9283-7. doi: 10.1128/JVI.01047-08. Epub 2008 Jul 9. J Virol. 2008. PMID: 18614638 Free PMC article.
-
Diversifying T-cell responses: safeguarding against pandemic influenza with mosaic nucleoprotein.J Virol. 2025 Mar 18;99(3):e0086724. doi: 10.1128/jvi.00867-24. Epub 2025 Feb 3. J Virol. 2025. PMID: 39898643 Free PMC article.
-
Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine.J Virol. 2021 May 24;95(12):e00507-21. doi: 10.1128/JVI.00507-21. Print 2021 May 24. J Virol. 2021. PMID: 33827939 Free PMC article.
-
A call to cellular & humoral arms: enlisting cognate T cell help to develop broad-spectrum vaccines against influenza A.Hum Vaccin. 2008 Mar-Apr;4(2):148-57. doi: 10.4161/hv.4.2.5169. Epub 2007 Oct 14. Hum Vaccin. 2008. PMID: 18382131 Review.
-
Review of 10 years of clinical experience with Chinese domestic trivalent influenza vaccine Anflu®.Hum Vaccin Immunother. 2014;10(1):73-82. doi: 10.4161/hv.26715. Epub 2013 Oct 8. Hum Vaccin Immunother. 2014. PMID: 24104060 Free PMC article. Review.
Cited by
-
Key Determinants of Cell-Mediated Immune Responses: A Randomized Trial of High Dose Vs. Standard Dose Split-Virus Influenza Vaccine in Older Adults.Front Aging. 2021 May;2:649110. doi: 10.3389/fragi.2021.649110. Epub 2021 May 21. Front Aging. 2021. PMID: 35128529 Free PMC article.
-
Regulation of antinucleoprotein IgG by systemic vaccination and its effect on influenza virus clearance.J Virol. 2011 May;85(10):5027-35. doi: 10.1128/JVI.00150-11. Epub 2011 Mar 2. J Virol. 2011. PMID: 21367900 Free PMC article.
-
The Way Forward: Potentiating Protective Immunity to Novel and Pandemic Influenza Through Engagement of Memory CD4 T Cells.J Infect Dis. 2019 Apr 8;219(Suppl_1):S30-S37. doi: 10.1093/infdis/jiy666. J Infect Dis. 2019. PMID: 30715376 Free PMC article.
-
Differences in Influenza-Specific CD4 T-Cell Mediated Immunity Following Acute Infection Versus Inactivated Vaccination in Children.J Infect Dis. 2021 Jun 15;223(12):2164-2173. doi: 10.1093/infdis/jiaa664. J Infect Dis. 2021. PMID: 33074330 Free PMC article.
-
Protection against H5N1 Influenza Virus Induced by Matrix-M Adjuvanted Seasonal Virosomal Vaccine in Mice Requires Both Antibodies and T Cells.PLoS One. 2015 Dec 22;10(12):e0145243. doi: 10.1371/journal.pone.0145243. eCollection 2015. PLoS One. 2015. PMID: 26696245 Free PMC article.
References
-
- Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2004 May 28;53(RR6):1–40. - PubMed
-
- Renfrey S, Watts A. Morphological and biochemical characterization of influenza vaccines commercially available in the United Kingdom. Vaccine. 1994 Jun;12(8):747–52. - PubMed
-
- Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine. 2002 Aug 19;20(25–26):3068–87. - PubMed
-
- Saikh KU, Tamura M, Kuwano K, Dai LC, West K, Ennis FA. Protective cross-reactive epitope on the nonstructural protein NS1 of influenza A virus. Viral immunology. 1993 Winter;6(4):229–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

