In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses
- PMID: 18977404
- PMCID: PMC2813682
- DOI: 10.1016/j.vaccine.2008.09.092
In vitro evidence that commercial influenza vaccines are not similar in their ability to activate human T cell responses
Abstract
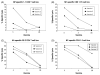
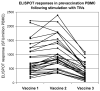
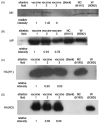
We evaluated three commercial trivalent inactivated vaccines (TIVs) from the 2007-2008 season in terms of their ability to elicit in vitro T cell responses. T cell-mediated immunity may offer a more cross-reactive vaccine approach for the prevention of pandemic or epidemic influenza. Human cytotoxic T cell lines demonstrated differences in matrix protein 1 and nucleocapsid protein recognition of autologous target cells. Peripheral blood mononuclear cells stimulated with each of the TIVs showed statistically significant differences between the vaccines in the numbers of IFNgamma producing cells activated. These data suggest that TIV vaccines are not similar in their ability to activate human T cell responses.
Figures

References
-
- Harper SA, Fukuda K, Uyeki TM, Cox NJ, Bridges CB. Prevention and control of influenza: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2004 May 28;53(RR6):1–40. - PubMed
-
- Renfrey S, Watts A. Morphological and biochemical characterization of influenza vaccines commercially available in the United Kingdom. Vaccine. 1994 Jun;12(8):747–52. - PubMed
-
- Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine. 2002 Aug 19;20(25–26):3068–87. - PubMed
-
- Saikh KU, Tamura M, Kuwano K, Dai LC, West K, Ennis FA. Protective cross-reactive epitope on the nonstructural protein NS1 of influenza A virus. Viral immunology. 1993 Winter;6(4):229–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

