In vitro susceptibility of established biofilms composed of a clinical wound isolate of Pseudomonas aeruginosa treated with lactoferrin and xylitol
- PMID: 18977641
- PMCID: PMC7008006
- DOI: 10.1016/j.ijantimicag.2008.08.013
In vitro susceptibility of established biofilms composed of a clinical wound isolate of Pseudomonas aeruginosa treated with lactoferrin and xylitol
Abstract
The medical impact of bacterial biofilms has increased with the recognition of biofilms as a major contributor to chronic wounds such as diabetic foot ulcers, venous leg ulcers and pressure ulcers. Traditional methods of treatment have proven ineffective, therefore this article presents in vitro evidence to support the use of novel antimicrobials in the treatment of Pseudomonas aeruginosa biofilm. An in vitro biofilm model with a clinical isolate of P. aeruginosa was subjected to treatment with either lactoferrin or xylitol alone or in combination. Combined lactoferrin and xylitol treatment disrupted the structure of the P. aeruginosa biofilm and resulted in a >2log reduction in viability. In situ analysis indicated that while xylitol treatment appeared to disrupt the biofilm structure, lactoferrin treatment resulted in a greater than two-fold increase in the number of permeabilised bacterial cells. The findings presented here indicated that combined treatment with lactoferrin and xylitol significantly decreases the viability of established P. aeruginosa biofilms in vitro and that the antimicrobial mechanism of this treatment includes both biofilm structural disruption and permeablisation of bacterial membranes.
Conflict of interest statement
Figures


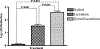

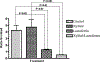
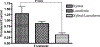

References
-
- James GA, Swogger E, Wolcott R, Pulcini ED, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound Repair Regen 2008;16:37–44. - PubMed
-
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diab Care 2008;31:596–615. Erratum in: Diab Care 2008;31:1271. - PubMed
-
- Gottrup F A specialized wound-healing center concept: importance of a multidisciplinary department structure and surgical treatment facilities in the treatment of chronic wounds. Am J Surg 2004;187(5A):38S–43S. - PubMed
-
- McGuckin M, Goldman R, Bolton L, Salcido R. The clinical relevance of microbiology in acute and chronic wounds. Adv Skin Wound Care 2003;16:12–23, quiz 24–5. - PubMed
-
- Bjarnsholt T, Kirketerp-Moller K, Jensen PO, Madsen KG, Phipps R, Krogfelt K, et al. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen 2008;16:2–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

