Transcriptional activation in yeast in response to copper deficiency involves copper-zinc superoxide dismutase
- PMID: 18977757
- PMCID: PMC2610518
- DOI: 10.1074/jbc.M807027200
Transcriptional activation in yeast in response to copper deficiency involves copper-zinc superoxide dismutase
Abstract
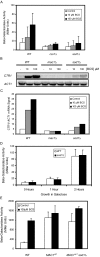
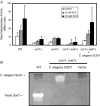
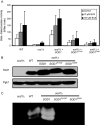
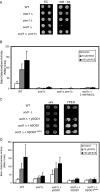
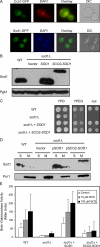
Copper is an essential trace element, yet excess copper can lead to membrane damage, protein oxidation, and DNA cleavage. To balance the need for copper with the necessity to prevent accumulation to toxic levels, cells have evolved sophisticated mechanisms to regulate copper acquisition, distribution, and storage. In Saccharomyces cerevisiae, transcriptional responses to copper deficiency are mediated by the copper-responsive transcription factor Mac1. Although Mac1 activates the transcription of genes involved in high affinity copper uptake during periods of deficiency, little is known about the mechanisms by which Mac1 senses or responds to reduced copper availability. Here we show that the copper-dependent enzyme Sod1 (Cu,Zn-superoxide dismutase) and its intracellular copper chaperone Ccs1 function in the activation of Mac1 in response to an external copper deficiency. Genetic ablation of either CCS1 or SOD1 results in a severe defect in the ability of yeast cells to activate the transcription of Mac1 target genes. The catalytic activity of Sod1 is essential for Mac1 activation and promotes a regulated increase in binding of Mac1 to copper response elements in the promoter regions of genomic Mac1 target genes. Although there is precedent for additional roles of Sod1 beyond protection of the cell from oxygen radicals, the involvement of this protein in copper-responsive transcriptional regulation has not previously been observed. Given the presence of both Sod1 and copper-responsive transcription factors in higher eukaryotes, these studies may yield important insights into how copper deficiency is sensed and appropriate cellular responses are coordinated.
Figures


Similar articles
-
Nitric oxide-mediated antioxidative mechanism in yeast through the activation of the transcription factor Mac1.PLoS One. 2014 Nov 25;9(11):e113788. doi: 10.1371/journal.pone.0113788. eCollection 2014. PLoS One. 2014. PMID: 25423296 Free PMC article.
-
The essential liaison of two copper proteins: the Cu-sensing transcription factor Mac1 and the Cu/Zn superoxide dismutase Sod1 in Saccharomyces cerevisiae.Curr Genet. 2023 Feb;69(1):41-53. doi: 10.1007/s00294-022-01258-8. Epub 2022 Dec 1. Curr Genet. 2023. PMID: 36456733
-
The yeast copper chaperone for copper-zinc superoxide dismutase (CCS1) is a multifunctional chaperone promoting all levels of SOD1 maturation.J Biol Chem. 2019 Feb 8;294(6):1956-1966. doi: 10.1074/jbc.RA118.005283. Epub 2018 Dec 10. J Biol Chem. 2019. PMID: 30530491 Free PMC article.
-
A copper connection to the uptake of platinum anticancer drugs.Proc Natl Acad Sci U S A. 2002 Oct 29;99(22):13963-5. doi: 10.1073/pnas.232574299. Epub 2002 Oct 21. Proc Natl Acad Sci U S A. 2002. PMID: 12391309 Free PMC article. Review. No abstract available.
-
Copper-regulatory domain involved in gene expression.Prog Nucleic Acid Res Mol Biol. 1998;58:165-95. doi: 10.1016/s0079-6603(08)60036-7. Prog Nucleic Acid Res Mol Biol. 1998. PMID: 9308366 Review.
Cited by
-
Human copper homeostasis: a network of interconnected pathways.Curr Opin Chem Biol. 2010 Apr;14(2):211-7. doi: 10.1016/j.cbpa.2010.01.003. Epub 2010 Feb 1. Curr Opin Chem Biol. 2010. PMID: 20117961 Free PMC article. Review.
-
Hac1 function revealed by the protein expression profile of a OtHAC1 mutant of thermotolerant methylotrophic yeast Ogataea thermomethanolica.Mol Biol Rep. 2018 Oct;45(5):1311-1319. doi: 10.1007/s11033-018-4287-4. Epub 2018 Jul 31. Mol Biol Rep. 2018. PMID: 30066298
-
Extra-mitochondrial Cu/Zn superoxide dismutase (Sod1) is dispensable for protection against oxidative stress but mediates peroxide signaling in Saccharomyces cerevisiae.Redox Biol. 2019 Feb;21:101064. doi: 10.1016/j.redox.2018.11.022. Epub 2018 Dec 1. Redox Biol. 2019. PMID: 30576923 Free PMC article.
-
Nitric oxide-mediated antioxidative mechanism in yeast through the activation of the transcription factor Mac1.PLoS One. 2014 Nov 25;9(11):e113788. doi: 10.1371/journal.pone.0113788. eCollection 2014. PLoS One. 2014. PMID: 25423296 Free PMC article.
-
SOD1 integrates signals from oxygen and glucose to repress respiration.Cell. 2013 Jan 17;152(1-2):224-35. doi: 10.1016/j.cell.2012.11.046. Cell. 2013. PMID: 23332757 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

