Coordination of two sequential ester-transfer reactions: exogenous guanosine binding promotes the subsequent omegaG binding to a group I intron
- PMID: 18978026
- PMCID: PMC2588497
- DOI: 10.1093/nar/gkn824
Coordination of two sequential ester-transfer reactions: exogenous guanosine binding promotes the subsequent omegaG binding to a group I intron
Abstract
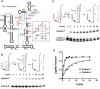
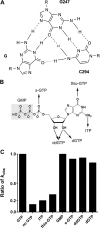
Self-splicing of group I introns is accomplished by two sequential ester-transfer reactions mediated by sequential binding of two different guanosine ligands, but it is yet unclear how the binding is coordinated at a single G-binding site. Using a three-piece trans-splicing system derived from the Candida intron, we studied the effect of the prior GTP binding on the later omegaG binding by assaying the ribozyme activity in the second reaction. We showed that adding GTP simultaneously with and prior to the esterified omegaG in a substrate strongly accelerated the second reaction, suggesting that the early binding of GTP facilitates the subsequent binding of omegaG. GTP-mediated facilitation requires C2 amino and C6 carbonyl groups on the Watson-Crick edge of the base but not the phosphate or sugar groups, suggesting that the base triple interactions between GTP and the binding site are important for the subsequent omegaG binding. Strikingly, GTP binding loosens a few local structures of the ribozyme including that adjacent to the base triple, providing structural basis for a rapid exchange of omegaG for bound GTP.
Figures




References
-
- Cech TR. Self-splicing of group I introns. Annu. Rev. Biochem. 1990;59:543–568. - PubMed
-
- Doudna JA, Cech TR. The chemical repertoire of natural ribozymes. Nature. 2002;418:222–228. - PubMed
-
- Narlikar GJ, Herschlag D. Mechanistic aspects of enzymatic catalysis: lessons from comparison of RNA and protein enzymes. Annu. Rev. Biochem. 1997;66:19–59. - PubMed
-
- Thirumalai D, Lee N, Woodson SA, Klimov D. Early events in RNA folding. Annu. Rev. Phys. Chem. 2001;52:751–762. - PubMed
-
- Treiber DK, Williamson JR. Beyond kinetic traps in RNA folding. Curr. Opin. Struct. Biol. 2001;11:309–314. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

