Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles
- PMID: 18978032
- PMCID: PMC2582270
- DOI: 10.1073/pnas.0809154105
Targeted delivery of cisplatin to prostate cancer cells by aptamer functionalized Pt(IV) prodrug-PLGA-PEG nanoparticles
Abstract
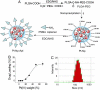
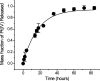
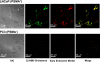
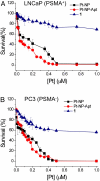
Cisplatin is used to treat a variety of tumors, but dose limiting toxicities or intrinsic and acquired resistance limit its application in many types of cancer including prostate. We report a unique strategy to deliver cisplatin to prostate cancer cells by constructing Pt(IV)-encapsulated prostate-specific membrane antigen (PSMA) targeted nanoparticles (NPs) of poly(D,L-lactic-co-glycolic acid) (PLGA)-poly(ethylene glycol) (PEG)-functionalized controlled release polymers. By using PLGA-b-PEG nanoparticles with PSMA targeting aptamers (Apt) on the surface as a vehicle for the platinum(IV) compound c,t,c-[Pt(NH(3))(2)(O(2)CCH(2)CH(2)CH(2)CH(2)CH(3))(2)Cl(2)] (1), a lethal dose of cisplatin was delivered specifically to prostate cancer cells. PSMA aptamer targeted delivery of Pt(IV) cargos to PSMA(+) LNCaP prostate cancer cells by endocytosis of the nanoparticle vehicles was demonstrated using fluorescence microscopy by colocalization of green fluorescent labeled cholesterol-encapsulated NPs and early endosome marker EEA-1. The choice of linear hexyl chains in 1 was the result of a systematic study to optimize encapsulation and controlled release from the polymer without compromising either feature. Release of cisplatin from the polymeric nanoparticles after reduction of 1 and formation of cisplatin 1,2-intrastrand d(GpG) cross-links on nuclear DNA was confirmed by using a monoclonal antibody for the adduct. A comparison between the cytotoxic activities of Pt(IV)-encapsulated PLGA-b-PEG NPs with the PSMA aptamer on the surface (Pt-NP-Apt), cisplatin, and the nontargeted Pt(IV)-encapsulated NPs (Pt-NP) against human prostate PSMA-overexpressing LNCaP and PSMA(-) PC3 cancer cells revealed significant differences. The effectiveness of PSMA targeted Pt-NP-Apt nanoparticles against the PSMA(+) LNCaP cells is approximately an order of magnitude greater than that of free cisplatin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
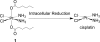
References
-
- Jemal A, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
-
- Israeli RS, Powell CT, Fair WR, Heston WDW. Molecular cloning of a complementary DNA encoding a prostate-specific membrane antigen. Cancer Res. 1993;53:227–230. - PubMed
-
- Murphy GP, et al. Current evaluation of the tissue localization and diagnostic utility of prostate specific membrane antigen. Cancer. 1998;83:2259–2269. - PubMed
-
- Kawakami M, Nakayama J. Enhanced expression of prostate-specific membrane antigen gene in prostate cancer as revealed by in situ hybridization. Cancer Res. 1997;57:2321–2324. - PubMed
-
- Wright GL, Jr, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–334. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

