Arrhythmogenic actions of the Ca2+ channel agonist FPL-64716 in Langendorff-perfused murine hearts
- PMID: 18978037
- PMCID: PMC2705814
- DOI: 10.1113/expphysiol.2008.044669
Arrhythmogenic actions of the Ca2+ channel agonist FPL-64716 in Langendorff-perfused murine hearts
Abstract
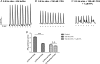
The experiments explored the extent to which alterations in L-type Ca(2+) channel-mediated Ca(2+) entry triggers Ca(2+)-mediated arrhythmogenesis in Langendorff-perfused murine hearts through use of the specific L-type Ca(2+) channel modulator FPL-64716 (FPL). Introduction of FPL (1 microm) resulted in a gradual development (>10 min) of diastolic electrical events and alternans in spontaneously beating hearts from which monophasic action potentials were recorded. In regularly paced hearts, they additionally led to non-sustained and sustained ventricular tachycardia (nsVT and sVT). Programmed electrical stimulation (PES) resulted in nsVT and sVT after 5-10 and >10 min perfusion, respectively. Pretreatments with nifedipine, diltiazem and cyclopiazonic acid abolished arrhythmogenic tendency induced by subsequent introduction of FPL, consistent with its dependence upon both extracellular Ca(2+) entry and the degree of filling of the sarcoplasmic reticular Ca(2+) store. Values for action potential duration at 90% repolarization when any of these agents were applied to FPL-treated hearts became indistinguishable from those shown by untreated control hearts, in contrast to earlier reports of their altering in long QT syndrome type 3 and hypokalaemic murine models for re-entrant arrhythmogenesis. These arrhythmic effects instead correlated with alterations in Ca(2+) homeostasis at the single-cell level found in investigations of the effects of both FPL and the same agents in regularly stimulated fluo-3 loaded myocytes. These findings are compatible with a prolonged extracellular Ca(2+) entry that potentially results in an intracellular Ca(2+) overload and produces the cardiac arrhythmogenecity following addition of FPL.
Figures




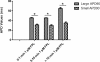



Similar articles
-
Acute atrial arrhythmogenesis in murine hearts following enhanced extracellular Ca(2+) entry depends on intracellular Ca(2+) stores.Acta Physiol (Oxf). 2010 Feb;198(2):143-58. doi: 10.1111/j.1748-1716.2009.02055.x. Epub 2009 Nov 3. Acta Physiol (Oxf). 2010. PMID: 19886909 Free PMC article.
-
Pharmacological changes in cellular Ca2+ homeostasis parallel initiation of atrial arrhythmogenesis in murine Langendorff-perfused hearts.Clin Exp Pharmacol Physiol. 2009 Oct;36(10):969-80. doi: 10.1111/j.1440-1681.2009.05170.x. Epub 2009 Mar 2. Clin Exp Pharmacol Physiol. 2009. PMID: 19298534 Free PMC article.
-
Nifedipine and diltiazem suppress ventricular arrhythmogenesis and calcium release in mouse hearts.Pflugers Arch. 2004 Nov;449(2):150-8. doi: 10.1007/s00424-004-1321-2. Epub 2004 Jul 30. Pflugers Arch. 2004. PMID: 15290304
-
L-type calcium channels.Curr Pharm Des. 2006;12(4):443-57. doi: 10.2174/138161206775474503. Curr Pharm Des. 2006. PMID: 16472138 Review.
-
Direct Estimation of CaV1.2 Gating Parameters: Quantification of Voltage Sensor - Pore Transductions and their Modulation by FLP 64176.Curr Mol Pharmacol. 2015;8(1):87-94. doi: 10.2174/1874467208666150507100256. Curr Mol Pharmacol. 2015. PMID: 25966699 Review.
Cited by
-
Cardiac Rhythm and Molecular Docking Studies of Ion Channel Ligands with Cardiotoxicity in Zebrafish.Cells. 2019 Jun 10;8(6):566. doi: 10.3390/cells8060566. Cells. 2019. PMID: 31185584 Free PMC article.
-
Murine Electrophysiological Models of Cardiac Arrhythmogenesis.Physiol Rev. 2017 Jan;97(1):283-409. doi: 10.1152/physrev.00007.2016. Physiol Rev. 2017. PMID: 27974512 Free PMC article. Review.
-
Early afterdepolarisation tendency as a simulated pro-arrhythmic risk indicator.Toxicol Res (Camb). 2017 Nov 1;6(6):912-921. doi: 10.1039/c7tx00141j. Epub 2017 Sep 14. Toxicol Res (Camb). 2017. PMID: 29456831 Free PMC article.
-
Epac-induced ryanodine receptor type 2 activation inhibits sodium currents in atrial and ventricular murine cardiomyocytes.Clin Exp Pharmacol Physiol. 2018 Mar;45(3):278-292. doi: 10.1111/1440-1681.12870. Epub 2017 Dec 7. Clin Exp Pharmacol Physiol. 2018. PMID: 29027245 Free PMC article.
-
Inhibition of Contractility of Isolated Caprine Detrusor by the Calcium Channel Blocker Cilnidipine and Reversal by Calcium Channel Openers.Curr Ther Res Clin Exp. 2023 Sep 30;99:100717. doi: 10.1016/j.curtheres.2023.100717. eCollection 2023. Curr Ther Res Clin Exp. 2023. PMID: 37869401 Free PMC article.
References
-
- Badaoui A, Huchet-Cadiou C, Leoty C. Effects of cyclopiazonic acid on membrane currents, contraction and intracellular calcium transients in frog heart. J Mol Cell Cardiol. 1995;27:2495–2505. - PubMed
-
- Balasubramaniam R, Chawla S, Grace AA, Huang CL-H. Caffeine-induced arrhythmias in murine hearts parallel changes in cellular Ca2+ homeostasis. Am J Physiol Heart Circ Physiol. 2005;289:H1584–H1593. - PubMed
-
- Balasubramaniam R, Chawla S, Mackenzie L, Schwiening CJ, Grace AA, Huang CL-H. Nifedipine and diltiazem suppress ventricular arrhythmogenesis and calcium release in mouse hearts. Pflugers Arch. 2004;449:150–158. - PubMed
-
- Baudet S, Shaoulian R, Bers DM. Effects of thapsigargin and cyclopiazonic acid on twitch force and sarcoplasmic reticulum Ca2+ content of rabbit ventricular muscle. Circ Res. 1993;73:813–819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

