Adaptation to chronic hypoxia involves immune cell invasion and increased expression of inflammatory cytokines in rat carotid body
- PMID: 18978039
- PMCID: PMC2643993
- DOI: 10.1152/ajplung.90383.2008
Adaptation to chronic hypoxia involves immune cell invasion and increased expression of inflammatory cytokines in rat carotid body
Abstract
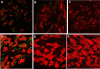
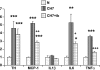
Exposure to chronic hypoxia (CH; 3-28 days at 380 Torr) induces adaptation in mammalian carotid body such that following CH an acute hypoxic challenge elicits an abnormally large increase in carotid sinus nerve impulse activity. The current study examines the hypothesis that CH initiates an immune response in the carotid body and that chemoreceptor hyperexcitability is dependent on the expression and action of inflammatory cytokines. CH resulted in a robust invasion of ED1(+) macrophages, which peaked on day 3 of exposure. Gene expression of proinflammatory cytokines, IL-1beta, TNFalpha, and the chemokine, monocyte chemoattractant protein-1, was increased >2-fold after 1 day of hypoxia followed by a >2-fold increase in IL-6 on day 3. After 28 days of CH, IL-6 remained elevated >5-fold, whereas expression of other cytokines recovered to normal levels. Cytokine expression was not restricted to immune cells. Studies of cultured type I cells harvested following 1 day of in vivo hypoxia showed elevated transcript levels of inflammatory cytokines. In situ hybridization studies confirmed expression of IL-6 in type I cells and also showed that CH induces IL-6 expression in supporting type II cells. Concurrent treatment of CH rats with anti-inflammatory drugs (ibuprofen or dexamethasone) blocked immune cell invasion and severely reduced CH-induced cytokine expression in carotid body. Drug treatment also blocked the development of chemoreceptor hypersensitivity in CH animals. Our findings indicate that chemoreceptor adaptation involves novel neuroimmune mechanisms, which may alter the functional phenotypes of type I cells and chemoafferent neurons.
Figures

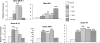

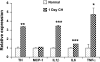


Comment in
-
Adaptation to chronic hypoxia involves immune cell invasion and increased expression of inflammatory cytokines in rat carotid body.Am J Physiol Lung Cell Mol Physiol. 2009 Feb;296(2):L156-7. doi: 10.1152/ajplung.90597.2008. Epub 2008 Dec 12. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19074555 Free PMC article. No abstract available.
References
-
- Bee D, Pallot DJ, Barer GR. Division of type I and endothelial cells in the hypoxic rat carotid body. Acta Anat (Basel) 126: 226–229, 1986. - PubMed
-
- Bisgard GE Carotid body mechanisms in acclimatization to hypoxia. Respir Physiol 121: 237–246, 2000. - PubMed
-
- Chen J, He L, Dinger B, Stensaas L, Fidone S. Role of endothelin and endothelin A-type receptor in physiological adaptation of the carotid body during chronic hypoxia. Am J Physiol Lung Cell Mol Physiol 282: L1314–L1323, 2002. - PubMed
-
- Chen J, He L, Dinger B, Stensaas L, Fidone S. Chronic hypoxia upregulates connexin43 expression in rat carotid body and petrosal ganglion. J Appl Physiol 92: 1480–1486, 2002. - PubMed
-
- Chen J, He L, Liu X, Dinger B, Stensaas L, Fidone S. Effect of the endothelin receptor antagonist bosentan on chronic hypoxia-induced morphological and physiological changes in rat carotid body. Am J Physiol Lung Cell Mol Physiol 292: L1257–L1262, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

