Preferential physical and functional interaction of pregnane X receptor with the SMRTalpha isoform
- PMID: 18978041
- PMCID: PMC2629818
- DOI: 10.1124/mol.108.047845
Preferential physical and functional interaction of pregnane X receptor with the SMRTalpha isoform
Abstract
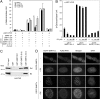
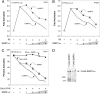
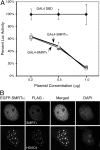
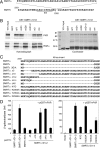
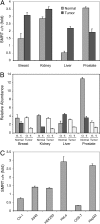
The silencing mediator for retinoid and thyroid hormone receptors (SMRT) serves as a platform for transcriptional repression elicited by several steroid/nuclear receptors and transcription factors. SMRT exists in two major splicing isoforms, alpha and tau, with SMRTalpha containing only an extra 46-amino acid sequence inserted immediately downstream from the C-terminal corepressor motif. Little is known about potential functional differences between these two isoforms. Here we show that the pregnane X receptor (PXR) interacts more strongly with SMRTalpha than with SMRTtau both in vitro and in vivo. It is interesting that the PXR-SMRTalpha interaction is also resistant to PXR ligand-induced dissociation, in contrast to the PXR-SMRTtau interaction. SMRTalpha consistently inhibits PXR activity more efficiently than does SMRTtau in transfection assays, although they possess comparable intrinsic repression activity and association with histone deacetylase. We further show that the mechanism for the enhanced PXR-SMRTalpha interaction involves both the 46-amino acid insert and the C-terminal corepressor motif. In particular, the first five amino acids of the SMRTalpha insert are essential and sufficient for the enhanced binding of SMRTalpha to PXR. Furthermore, we demonstrate that Tyr2354 and Asp2355 residues of the SMRTalpha insert are most critical for the enhanced interaction. In addition, expression data show that SMRTalpha is more abundantly expressed in most human tissues and cancer cell lines, and together these data suggest that SMRTalpha may play a more important role than SMRTtau in the negative regulation of PXR.
Figures

References
-
- Chen JD and Evans RM (1995) A transcriptional co-repressor that interacts with nuclear hormone receptors. Nature 377 454-457. - PubMed
-
- Falkner KC, Pinaire JA, Xiao GH, Geoghegan TE, and Prough RA (2001) Regulation of the rat glutathione S-transferase A2 gene by glucocorticoids: involvement of both the glucocorticoid and pregnane X receptors. Mol Pharmacol 60 611-619. - PubMed
-
- Gao YD, Olson SH, Balkovec JM, Zhu Y, Royo I, Yabut J, Evers R, Tan EY, Tang W, Hartley DP, et al. (2007) Attenuating pregnane X receptor (PXR) activation: a molecular modelling approach. Xenobiotica 37 124-138. - PubMed
-
- Geick A, Eichelbaum M, and Burk O (2001) Nuclear receptor response elements mediate induction of intestinal MDR1 by rifampin. J Biol Chem 276 14581-14587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

