Maximal efficiency of coupling between ATP hydrolysis and translocation of polypeptides mediated by SecB requires two protomers of SecA
- PMID: 18978043
- PMCID: PMC2632081
- DOI: 10.1128/JB.01321-08
Maximal efficiency of coupling between ATP hydrolysis and translocation of polypeptides mediated by SecB requires two protomers of SecA
Abstract
SecA is the ATPase that provides energy for translocation of precursor polypeptides through the SecYEG translocon in Escherichia coli during protein export. We showed previously that when SecA receives the precursor from SecB, the ternary complex is fully active only when two protomers of SecA are bound. Here we used variants of SecA and of SecB that populate complexes containing two protomers of SecA to different degrees to examine both the hydrolysis of ATP and the translocation of polypeptides. We conclude that the low activity of the complexes with only one protomer is the result of a low efficiency of coupling between ATP hydrolysis and translocation.
Figures
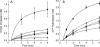
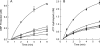


References
-
- Crane, J. M., C. Mao, A. A. Lilly, V. F. Smith, Y. Suo, W. L. Hubbell, and L. L. Randall. 2005. Mapping of the docking of SecA onto the chaperone SecB by site-directed spin labeling: insight into the mechanism of ligand transfer during protein export. J. Mol. Biol. 353295-307. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

