Heat-labile enterotoxin promotes Escherichia coli adherence to intestinal epithelial cells
- PMID: 18978047
- PMCID: PMC2612432
- DOI: 10.1128/JB.00822-08
Heat-labile enterotoxin promotes Escherichia coli adherence to intestinal epithelial cells
Abstract
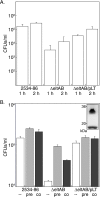
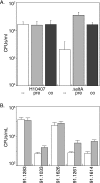
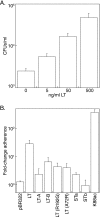
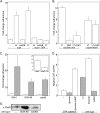
Given recent evidence suggesting that the heat-labile enterotoxin (LT) provides a colonization advantage for enterotoxigenic Escherichia coli (ETEC) in vivo, we hypothesized that LT preconditions the host intestinal epithelium for ETEC adherence. To test this hypothesis, we used an in vitro model of ETEC adherence to examine the role of LT in promoting bacterium-host interactions. We present data demonstrating that elaboration of LT promotes a significant increase in E. coli adherence. This phenotype is primarily dependent on the inherent ADP-ribosylation activity of this toxin, with a secondary role observed for the receptor-binding LT-B subunit. Rp-3',5'-cyclic AMP (cAMP), an inhibitor of protein kinase A, was sufficient to abrogate LT's ability to promote subsequent bacterial adherence. Increased adherence was not due to changes in the surface expression of the host receptor for the K88ac adhesin. Evidence is also presented for a role for bacterial sensing of host-derived cAMP in promoting adherence to host cells.
Figures

References
-
- Baker, D. R., L. O. Billey, and D. H. Francis. 1997. Distribution of K88 Escherichia coli-adhesive and nonadhesive phenotypes among pigs of four breeds. Vet. Microbiol. 54123-132. - PubMed
-
- Bakker, D., P. T. Willemsen, L. H. Simons, F. G. van Zijderveld, and F. K. de Graaf. 1992. Characterization of the antigenic and adhesive properties of FaeG, the major subunit of K88 fimbriae. Mol. Microbiol. 6247-255. - PubMed
-
- Berberov, E. M., Y. Zhou, D. H. Francis, M. A. Scott, S. D. Kachman, and R. A. Moxley. 2004. Relative importance of heat-labile enterotoxin in the causation of severe diarrheal disease in the gnotobiotic piglet model by a strain of enterotoxigenic Escherichia coli that produces multiple enterotoxins. Infect. Immun. 723914-3924. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

