AglC and AglK are involved in biosynthesis and attachment of diacetylated glucuronic acid to the N-glycan in Methanococcus voltae
- PMID: 18978056
- PMCID: PMC2612417
- DOI: 10.1128/JB.00885-08
AglC and AglK are involved in biosynthesis and attachment of diacetylated glucuronic acid to the N-glycan in Methanococcus voltae
Abstract
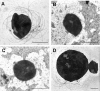
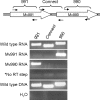
Recent advances in the field of prokaryotic N-glycosylation have established a foundation for the pathways and proteins involved in this important posttranslational protein modification process. To continue the study of the Methanococcus voltae N-glycosylation pathway, characteristics of known eukaryotic, bacterial, and archaeal proteins involved in the N-glycosylation process were examined and used to select candidate M. voltae genes for investigation as potential glycosyl transferase and flippase components. The targeted genes were knocked out via linear gene replacement, and the resulting effects on N-glycan assembly were identified through flagellin and surface (S) layer protein glycosylation defects. This study reports the finding that deletion of two putative M. voltae glycosyl transferase genes, designated aglC (for archaeal glycosylation) and aglK, interfered with proper N-glycosylation. This resulted in flagellin and S-layer proteins with significantly reduced apparent molecular masses, loss of flagellar assembly, and absence of glycan attachment. Given previous knowledge of both the N-glycosylation pathway in M. voltae and the general characteristics of N-glycosylation components, it appears that AglC and AglK are involved in the biosynthesis or transfer of diacetylated glucuronic acid within the glycan structure. In addition, a knockout of the putative flippase candidate gene (Mv891) had no effect on N-glycosylation but did result in the production of giant cells with diameters three to four times that of wild-type cells.
Figures






Similar articles
-
Identification of genes involved in the biosynthesis and attachment of Methanococcus voltae N-linked glycans: insight into N-linked glycosylation pathways in Archaea.Mol Microbiol. 2006 Jul;61(1):259-68. doi: 10.1111/j.1365-2958.2006.05226.x. Mol Microbiol. 2006. PMID: 16824110
-
Identification of genes involved in the assembly and attachment of a novel flagellin N-linked tetrasaccharide important for motility in the archaeon Methanococcus maripaludis.Mol Microbiol. 2009 May;72(3):633-44. doi: 10.1111/j.1365-2958.2009.06671.x. Mol Microbiol. 2009. PMID: 19400781
-
Characterization of flagellum gene families of methanogenic archaea and localization of novel flagellum accessory proteins.J Bacteriol. 2001 Dec;183(24):7154-64. doi: 10.1128/JB.183.24.7154-7164.2001. J Bacteriol. 2001. PMID: 11717274 Free PMC article.
-
Archaeal flagella, bacterial flagella and type IV pili: a comparison of genes and posttranslational modifications.J Mol Microbiol Biotechnol. 2006;11(3-5):167-91. doi: 10.1159/000094053. J Mol Microbiol Biotechnol. 2006. PMID: 16983194 Review.
-
Hot and sweet: protein glycosylation in Crenarchaeota.Biochem Soc Trans. 2013 Feb 1;41(1):384-92. doi: 10.1042/BST20120296. Biochem Soc Trans. 2013. PMID: 23356316 Review.
Cited by
-
S-layer glycoproteins and flagellins: reporters of archaeal posttranslational modifications.Archaea. 2010 Jul 20;2010:612948. doi: 10.1155/2010/612948. Archaea. 2010. PMID: 20721273 Free PMC article. Review.
-
Overview of the genetic tools in the Archaea.Front Microbiol. 2012 Oct 2;3:337. doi: 10.3389/fmicb.2012.00337. eCollection 2012. Front Microbiol. 2012. PMID: 23060865 Free PMC article.
-
AglJ adds the first sugar of the N-linked pentasaccharide decorating the Haloferax volcanii S-layer glycoprotein.J Bacteriol. 2010 Nov;192(21):5572-9. doi: 10.1128/JB.00705-10. Epub 2010 Aug 27. J Bacteriol. 2010. PMID: 20802039 Free PMC article.
-
Genomic variation and biogeography of Antarctic haloarchaea.Microbiome. 2018 Jun 20;6(1):113. doi: 10.1186/s40168-018-0495-3. Microbiome. 2018. PMID: 29925429 Free PMC article.
-
N-Glycosylation Is Important for Halobacterium salinarum Archaellin Expression, Archaellum Assembly and Cell Motility.Front Microbiol. 2019 Jun 18;10:1367. doi: 10.3389/fmicb.2019.01367. eCollection 2019. Front Microbiol. 2019. PMID: 31275283 Free PMC article.
References
-
- Abu-Qarn, M., and J. Eichler. 2006. Protein N-glycosylation in Archaea: defining Haloferax volcanii genes involved in S-layer glycoprotein glycosylation. Mol. Microbiol. 61511-525. - PubMed
-
- Abu-Qarn, M., S. Yurist-Doutsch, A. Giordano, A. Trauner, H. R. Morris, P. Hitchen, O. Medalia, A. Dell, and J. Eichler. 2007. Haloferax volcanii AglB and AglD are involved in N-glycosylation of the S-layer glycoprotein and proper assembly of the surface layer. J. Mol. Biol. 3741224-1236. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

