Sulfur transfer through an arbuscular mycorrhiza
- PMID: 18978070
- PMCID: PMC2613693
- DOI: 10.1104/pp.108.129866
Sulfur transfer through an arbuscular mycorrhiza
Abstract
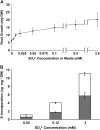
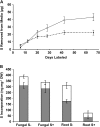
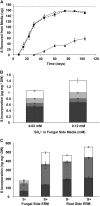
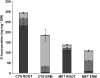
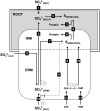
Despite the importance of sulfur (S) for plant nutrition, the role of the arbuscular mycorrhizal (AM) symbiosis in S uptake has received little attention. To address this issue, 35S-labeling experiments were performed on mycorrhizas of transformed carrot (Daucus carota) roots and Glomus intraradices grown monoxenically on bicompartmental petri dishes. The uptake and transfer of 35SO4(2-) by the fungus and resulting 35S partitioning into different metabolic pools in the host roots was analyzed when altering the sulfate concentration available to roots and supplying the fungal compartment with cysteine (Cys), methionine (Met), or glutathione. Additionally, the uptake, transfer, and partitioning of 35S from the reduced S sources [35S]Cys and [35S]Met was determined. Sulfate was taken up by the fungus and transferred to mycorrhizal roots, increasing root S contents by 25% in a moderate (not growth-limiting) concentration of sulfate. High sulfate levels in the mycorrhizal root compartment halved the uptake of 35SO4(2-) from the fungal compartment. The addition of 1 mm Met, Cys, or glutathione to the fungal compartment reduced the transfer of sulfate by 26%, 45%, and 80%, respectively, over 1 month. Similar quantities of 35S were transferred to mycorrhizal roots whether 35SO4(2-), [35S]Cys, or [35S]Met was supplied in the fungal compartment. Fungal transcripts for putative S assimilatory genes were identified, indicating the presence of the trans-sulfuration pathway. The suppression of fungal sulfate transfer in the presence of Cys coincided with a reduction in putative sulfate permease and not sulfate adenylyltransferase transcripts, suggesting a role for fungal transcriptional regulation in S transfer to the host. A testable model is proposed describing root S acquisition through the AM symbiosis.
Figures


References
-
- Banerjee R, Evande R, Kabil O, Ojha S, Taoka S (2003) Reaction mechanism and regulation of cystathionine beta-synthase. Biochim Biophys Acta 1647 30–35 - PubMed
-
- Baumgardner RE, Lavery TF, Rogers CM, Isil SS (2002) Estimates of the atmospheric deposition of sulfur and nitrogen species: Clean Air Status and Trends Network, 1990-2000. Environ Sci Technol 36 2614–2629 - PubMed
-
- Bhat KKS, Nye PH, Baldwin JP (1976) Diffusion of phosphate to plant roots in soil. 4. Concentration distance profile in rhizosphere of roots with root hairs in a low-p soil. Plant Soil 44 63–72
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

