Functional characterization of a higher plant sphingolipid Delta4-desaturase: defining the role of sphingosine and sphingosine-1-phosphate in Arabidopsis
- PMID: 18978071
- PMCID: PMC2613699
- DOI: 10.1104/pp.108.129411
Functional characterization of a higher plant sphingolipid Delta4-desaturase: defining the role of sphingosine and sphingosine-1-phosphate in Arabidopsis
Abstract
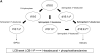
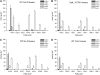
The role of Delta4-unsaturated sphingolipid long-chain bases such as sphingosine was investigated in Arabidopsis (Arabidopsis thaliana). Identification and functional characterization of the sole Arabidopsis ortholog of the sphingolipid Delta4-desaturase was achieved by heterologous expression in Pichia pastoris. A P. pastoris mutant disrupted in the endogenous sphingolipid Delta4-desaturase gene was unable to synthesize glucosylceramides. Synthesis of glucosylceramides was restored by the expression of Arabidopsis gene At4g04930, and these sphingolipids were shown to contain Delta4-unsaturated long-chain bases, confirming that this open reading frame encodes the sphingolipid Delta4-desaturase. At4g04930 has a very restricted expression pattern, transcripts only being detected in pollen and floral tissues. Arabidopsis insertion mutants disrupted in the sphingolipid Delta4-desaturase At4g04930 were isolated and found to be phenotypically normal. Sphingolipidomic profiling of a T-DNA insertion mutant indicated the absence of Delta4-unsaturated sphingolipids in floral tissue, also resulting in the reduced accumulation of glucosylceramides. No difference in the response to drought or water loss was observed between wild-type plants and insertion mutants disrupted in the sphingolipid Delta4-desaturase At4g04930, nor was any difference observed in stomatal closure after treatment with abscisic acid. No differences in pollen viability between wild-type plants and insertion mutants were detected. Based on these observations, it seems unlikely that Delta4-unsaturated sphingolipids and their metabolites such as sphingosine-1-phosphate play a significant role in Arabidopsis growth and development. However, Delta4-unsaturated ceramides may play a previously unrecognized role in the channeling of substrates for the synthesis of glucosylceramides.
Figures

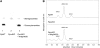





References
-
- Alonso JM, Stepanova AN, Leisse TJ, Kim CJ, Chen H, Shinn P, Stevenson DK, Zimmerman J, Barajas P, Cheuk R, et al (2003) Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301 653–657 - PubMed
-
- Brownlee C (2001) Intracellular signalling: sphingosine-1-phosphate branches out. Curr Biol 11 R535–R538 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

