Differentiation potential of multipotent progenitor cells derived from war-traumatized muscle tissue
- PMID: 18978407
- PMCID: PMC2657299
- DOI: 10.2106/JBJS.H.00049
Differentiation potential of multipotent progenitor cells derived from war-traumatized muscle tissue
Abstract
Background: Recent military conflicts have resulted in numerous extremity injuries requiring complex orthopaedic reconstructive procedures, which begin with a thorough débridement of all contaminated and necrotic tissue in the zone of injury. The site of injury is also the site of healing, and we propose that débrided muscle tissue contains cells with robust reparative and regenerative potential.
Methods: Débrided muscle from soldiers who had sustained traumatic open extremity injuries was collected during surgical débridement procedures at Walter Reed Army Medical Center. With modifications to a previously described stem-cell-isolation protocol, mesenchymal progenitor cells were harvested from traumatized muscle, enriched, expanded in culture, and exposed to induction media for osteogenesis, adipogenesis, and chondrogenesis.
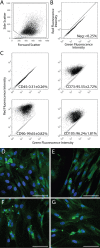
Results: The isolated mesenchymal progenitor cells stained positive for cell-surface markers (CD73, CD90, CD105), which are characteristic of adult human mesenchymal stem cells. Histological identification of lineage-specific markers demonstrated the potential of these cells to differentiate into multiple mesenchymal lineages. Reverse transcription-polymerase chain reaction analysis confirmed multilineage mesenchymal differentiation at the gene-expression level.
Conclusions: To our knowledge, the present report provides the first description of mesenchymal progenitor cell isolation from traumatized human muscle. These cells may play an integral role in tissue repair and regeneration and merit additional investigation as they could be useful in future cell-based tissue-engineering strategies.
Figures



References
-
- Jussila J. Measurement of kinetic energy dissipation with gelatine fissure formation with special reference to gelatine validation. Forensic Sci Int. 2005;150:53-62. - PubMed
-
- Jussila J, Kjellstrom BT, Leppaniemi A. Ballistic variables and tissue devitalisation in penetrating injury—establishing relationship through meta-analysis of a number of pig tests. Injury. 2005;36:282-92. - PubMed
-
- Williams AJ, Hartings JA, Lu XC, Rolli ML, Tortella FC. Penetrating ballistic-like brain injury in the rat: differential time courses of hemorrhage, cell death, inflammation, and remote degeneration. J Neurotrauma. 2006;23:1828-46. - PubMed
-
- Caterson EJ, Nesti LJ, Danielson KG, Tuan RS. Human marrow-derived mesenchymal progenitor cells: isolation, culture expansion, and analysis of differentiation. Mol Biotechnol. 2002;20:245-56. - PubMed
-
- Noth U, Osyczka AM, Tuli R, Hickok NJ, Danielson KG, Tuan RS. Multilineage mesenchymal differentiation potential of human trabecular bone-derived cells. J Orthop Res. 2002;20:1060-9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

