Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling
- PMID: 18978795
- PMCID: PMC2744141
- DOI: 10.1038/ni.1666
Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling
Abstract
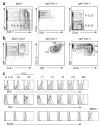
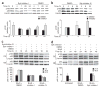
The survival of transitional and mature B cells requires both the B cell antigen receptor (BCR) and BLyS receptor 3 (BR3), which suggests that these receptors send signals that are nonredundant or that engage in crosstalk with each other. Here we show that BCR signaling induced production of the nonclassical transcription factor NF-kappaB pathway substrate p100, which is required for transmission of BR3 signals and thus B cell survival. The capacity for sustained p100 production emerged during transitional B cell differentiation, the stage at which BCR signals begin to mediate survival rather than negative selection. Our findings identify a molecular mechanism for the reliance of primary B cells on continuous BR3 and BCR signaling, as well as for the gradual resistance to negative selection that is acquired during B cell maturation.
Figures

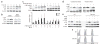
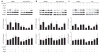

References
-
- Moore PA, et al. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. - PubMed
-
- Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. - PubMed
-
- Yan M, et al. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–1552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

