ICAM-2 expression mediates a membrane-actin link, confers a nonmetastatic phenotype and reflects favorable tumor stage or histology in neuroblastoma
- PMID: 18978946
- PMCID: PMC2575377
- DOI: 10.1371/journal.pone.0003629
ICAM-2 expression mediates a membrane-actin link, confers a nonmetastatic phenotype and reflects favorable tumor stage or histology in neuroblastoma
Abstract
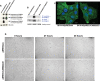
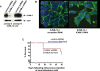
The actin cytoskeleton is a primary determinant of tumor cell motility and metastatic potential. Motility and metastasis are thought to be regulated, in large part, by the interaction of membrane proteins with cytoplasmic linker proteins and of these linker proteins, in turn, with actin. However, complete membrane-to-actin linkages have been difficult to identify. We used co-immunoprecipitation and competitive peptide assays to show that intercellular adhesion molecule-2 (ICAM-2)/alpha-actinin/actin may comprise such a linkage in neuroblastoma cells. ICAM-2 expression limited the motility of these cells and redistributed actin fibers in vitro, and suppressed development of disseminated tumors in an in vivo model of metastatic neuroblastoma. Consistent with these observations, immunohistochemical analysis demonstrated ICAM-2 expression in primary neuroblastoma tumors exhibiting features that are associated with limited metastatic disease and more favorable clinical outcome. In neuroblastoma cell lines, ICAM-2 expression did not affect AKT activation, tumorigenic potential or chemosensitivity, as has been reported for some types of transfected cells. The observed ICAM-2-mediated suppression of metastatic phenotype is a novel function for this protein, and the interaction of ICAM-2/alpha-actinin/actin represents the first complete membrane-linker protein-actin linkage to impact tumor cell motility in vitro and metastatic potential in an in vivo model. Current work focuses on identifying specific protein domains critical to the regulation of neuroblastoma cell motility and metastasis and on determining if these domains represent exploitable therapeutic targets.
Conflict of interest statement
Figures




References
-
- Bretscher A. Regulation of cortical structure by the ezrin-radixin-moesin protein family. Curr Opin Cell Biol. 1999;11:109–116. - PubMed
-
- Sun C-X, Robb VA, Gutmann DH. Protein 4.1 tumor suppressors: getting a FERM grip on growth regulation. J Cell Sci. 2002;2002:1153991–3400. - PubMed
-
- Gluck U, Ben-Ze'ev A. Modulation of alpha-actinin levels affects cell motility and confers tumorigenicity on 3T3 cells. J Cell Sci. 1994;107:1773–1782. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

