Intrascleral drug delivery to the eye using hollow microneedles
- PMID: 18979189
- PMCID: PMC2900774
- DOI: 10.1007/s11095-008-9756-3
Intrascleral drug delivery to the eye using hollow microneedles
Abstract
Purpose: This study tested the hypothesis that hollow microneedles can infuse solutions containing soluble molecules, nanoparticles, and microparticles into sclera in a minimally invasive manner.
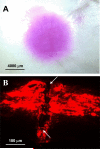
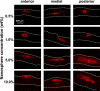
Methods: Individual hollow microneedles were inserted into, but not across, human cadaver sclera and aqueous solutions containing sulforhodamine or fluorescently tagged nanoparticles or microparticles were infused into sclera at constant pressure. The infused volume of fluid was measured and imaged histologically as a function of scleral thickness, infusion pressure, needle retraction depth and the presence of spreading enzymes (hyaluronidase and collagenase).
Results: Individual hollow microneedles were able to insert into sclera. Fluid infusion was extremely slow after microneedle insertion into the sclera without retraction, but partial retraction of the microneedle over a distance of 200-300 microm enabled infusion of 10-35 microl of fluid into the tissue. Scleral thickness and infusion pressure had insignificant effects on fluid delivery. Nanoparticle suspensions were also delivered into sclera, but microparticles were delivered only in the presence of hyaluronidase and collagenase spreading enzymes, which suggested the role of scleral glycosaminoglycans and collagen fibers as rate-limiting barriers.
Conclusion: This study shows that hollow microneedles can infuse solutions into the sclera for minimally invasive delivery of soluble molecules, nanoparticles and microparticles.
Figures

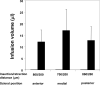

 ), 15 (
), 15 ( ), 20 (
), 20 ( ) and 25 (
) and 25 ( ) psi. Data are expressed as mean values (n ≥ 3) with standard deviation bars.
) psi. Data are expressed as mean values (n ≥ 3) with standard deviation bars.

References
-
- Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin Drug Deliv. 2006;3:275–87. - PubMed
-
- Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems. A shift to the posterior segment. Drug Discov Today. 2008;13:135–43. - PubMed
-
- Maurice DM. Drug delivery to the posterior segment from drops. Surv Ophthalmol. 2002;47(Suppl 1):S41–52. - PubMed
-
- Jager RD, Aiello LP, Patel SC, Cunningham ET., Jr. Risks of intravitreous injection: a comprehensive review. Retina. 2004;24:676–98. - PubMed
-
- Geroski DH, Edelhauser HF. Transscleral drug delivery for posterior segment disease. Adv Drug Deliv Rev. 2001;52:37–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

