Modification of quinone electrochemistry by the proteins in the biological electron transfer chains: examples from photosynthetic reaction centers
- PMID: 18979192
- PMCID: PMC2746407
- DOI: 10.1007/s10863-008-9179-1
Modification of quinone electrochemistry by the proteins in the biological electron transfer chains: examples from photosynthetic reaction centers
Abstract
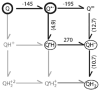
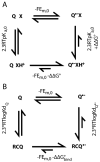
Quinones such as ubiquinone are the lipid soluble electron and proton carriers in the membranes of mitochondria, chloroplasts and oxygenic bacteria. Quinones undergo controlled redox reactions bound to specific sites in integral membrane proteins such as the cytochrome bc(1) oxidoreductase. The quinone reactions in bacterial photosynthesis are amongst the best characterized, presenting a model to understand how proteins modulate cofactor chemistry. The free energy of ubiquinone redox reactions in aqueous solution and in the Q(A) and Q(B) sites of the bacterial photosynthetic reaction centers (RCs) are compared. In the primary Q(A) site ubiquinone is reduced only to the anionic semiquinone (Q(*-)) while in the secondary Q(B) site the product is the doubly reduced, doubly protonated quinol (QH(2)). The ways in which the protein modifies the relative energy of each reduced and protonated intermediate are described. For example, the protein stabilizes Q(*-) while destabilizing Q(=) relative to aqueous solution through electrostatic interactions. In addition, kinetic and thermodynamic mechanisms for stabilizing the intermediate semiquinones are compared. Evidence for the protein sequestering anionic compounds by slowing both on and off rates as well as by binding the anion more tightly is reviewed.
Figures
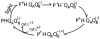

References
-
- Alexov EG, Gunner MR. Calculated protein and proton motions coupled to electron transfer: electron transfer from QA− to QB in bacterial photosynthetic reaction centers. Biochemistry. 1999;38:8253–8270. - PubMed
-
- Alexov E, Miksovska J, Baciou L, Schiffer M, Hanson DK, Sebban P, Gunner MR. Modeling the effects of mutations on the free energy of the first electron transfer from QA− to QB in photosynthetic reaction centers. Biochemistry. 2000;39:5940–5952. - PubMed
-
- Baker NA. Improving implicit solvent simulations: a Poisson-centric view. Cur Opin Struct Biol. 2005;15:137–143. - PubMed
-
- Bashford D, Karplus M. The pKas of ionizable groups in proteins: atomic detail from a continuum electrostatic model. Biochemistry. 1990;29:10219–10225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

