The proteoglycan osteoglycin/mimecan is correlated with arteriogenesis
- PMID: 18979232
- PMCID: PMC2758385
- DOI: 10.1007/s11010-008-9935-x
The proteoglycan osteoglycin/mimecan is correlated with arteriogenesis
Abstract
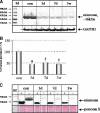
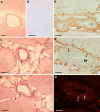
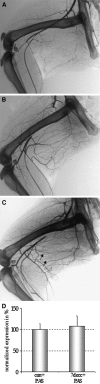
Arteriogenesis or collateral growth is able to compensate for the stenosis of major arteries. Using differential display RT-PCR on growing and quiescent collateral arteries in a rabbit femoral artery ligation model, we cloned the rabbit full-length cDNA of osteoglycin/mimecan. Osteoglycin was present in the adventitia of collateral arteries as a glycosylated protein without keratan sulfate side chains, mainly produced by smooth muscle cells (SMCs) and perivascular fibroblasts. Northern blot, Western blot, and immunohistochemistry confirmed a collateral artery-specific downregulation of osteoglycin from 6 h to 3 weeks after the onset of arteriogenesis. Treatment of primary SMCs with the arteriogenic protein fibroblast growth factor-2 (FGF-2) resulted in a similar reduction of osteoglycin expression as observed in vivo. Application of the FGF-2 inhibitor polyanethole sulfonic acid (PAS) blocked the downregulation of osteoglycin and interfered with arteriogenesis. From our study we conclude that downregulation of osteoglycin is a fundamental requirement for proper arteriogenesis.
Figures


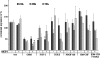
References
-
- WHO (1999) WHO Library Cataloguing in Publication Data. The world health report 1999: Making a difference I. Title: Making a difference ISBN 92 4 156194 7 (NLM Classification: WA 540.1) ISSN 1020-3311
-
- Schaper W, Piek JJ, Munoz-Chapuli R et al (1999) Collateral circulation of the heart. In: Ware JA, Simons M (eds) Angiogenesis and cardiovascular disease. Oxford University Press, New York, pp 159–198
-
- Deindl E, Ziegelhoffer T, Kanse SM et al (2003) Receptor-independent role of the urokinase-type plasminogen activator during arteriogenesis. FASEB J 17:1174–1176 - PubMed
-
- Ito WD, Arras M, Winkler B et al (1997) Angiogenesis but not collateral growth is associated with ischemia after femoral artery occlusion. Am J Physiol 273:H1255–H1265 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

