Second-order self-organization in coordination-driven self-assembly: exploring the limits of self-selection
- PMID: 18980302
- PMCID: PMC2650397
- DOI: 10.1021/ic801711q
Second-order self-organization in coordination-driven self-assembly: exploring the limits of self-selection
Abstract
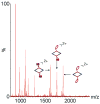
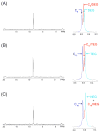
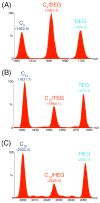
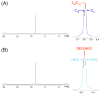
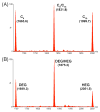
Self-organization during the self-assembly of a series of functionalized bispyridyl organic donors with complementary di-Pt(II) acceptors into supramolecular rhomboids and rectangles is explored. The connectivity and location of functional groups on the organic donors ensures that they do not interfere sterically or electronically with their respective binding sites. Carefully controlled reaction conditions are employed so that the only means of self-organization during self-assembly is through "second-order" effects arising from the distal functional groups themselves. With the selection of functionalized systems studied, the extent of second-order self-organization varies from essentially zero to quite pronounced.
Figures
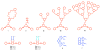






References
-
- Lehn JM. Angew Chem Int Ed. 1990;29:1304.
- Lindsey JS. New J Chem. 1991;15:153.
- Philp D, Stoddart JF. Angew Chem Int Ed. 1996;35:1154.
- Rebek J., Jr Acc Chem Res. 1999;32:278.
- Yaghi OM, O’Keeffe M, Ockwig NW, Chae HK, Eddaoudi M, Kim J. Nature. 2003;423:705. - PubMed
-
- Orr GW, Barbour LT, Atwood JL. Science. 1999;285:1049. - PubMed
- Storhoff JJ, Mirkin CA. Chem Rev. 1999;99:1849. - PubMed
- Lehn JM. Science. 2002;295:2400. - PubMed
- Lehn JM. Proc Natl Acad Sci USA. 2002;99:4763. - PMC - PubMed
- Lehn JM. Rep Prog Phys. 2004;67:249.
- Hollingsworth MD. Science. 2002;295:2410. - PubMed
-
- Deitrich B, Lehn JM, Sauvage JP. Tetrahedron Lett. 1969;34:2889.
- Pederson C. J Ger Offen. 1970. Application: DE 70-2015732.
- Lehn JM, Sauvage JP, Dietrich B. J Am Chem Soc. 1970;92:2916.
- Cram DJ, Cram JM. Acc Chem Res. 1978;11:8.
- Lehn JM. Acc Chem Res. 1978;11:49.
- Pederson CJ, Lehn JM, Cram DJ. Resonance. 2001;6:71.
-
- Gleick J. Chaos: Making a New Science. Penguin; New York, NY: 1988.
- Kauffman S. At Home in the Universe. Oxiford University Press; 1995.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

